Nuclear Reactors
- Page ID
- 1470
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A nuclear reactor is a device in which nuclear reactions are generated, and the chain reaction is controlled to release large amount of steady heat, thereby producing energy.
Introduction
Nuclear fission is the process in which the nucleus of an atom is split, forming nuclei of lighter atoms and neutrons. The mass of these products is less than the original mass. According to Einstein's equation \(E=mc^2\), the small amount of missing mass is converted into a large amount of energy.
A chain reaction occurs when the neutrons released in fission collide with at least one other nuclei, causing the fission of another nuclei. The process then repeats. In today's nuclear reactors, Uranium-235 is commonly used. During each U235/92 fission, 2.5 neutrons are released on average. Note here; Uranium 235 is used because it has a fairly large nucleus which facilitates the process of fission. An explosion could only occur if the reaction becomes uncontrolled. When one mass of U-235 exceeds the mass of U-235 that is large enough to hold down a chain reaction, also known as critical mass, an explosion occurs. A great example of this phenomenon would be a nuclear bomb. In uncontrolled reactions, neutrons escape too quickly to maintain a chain reaction. This rapid release of nuclear energy causes an explosion. However, in a nuclear reactor, energy is being produced at a controlled, constant rate; a nuclear explosion is unlikely to occur.
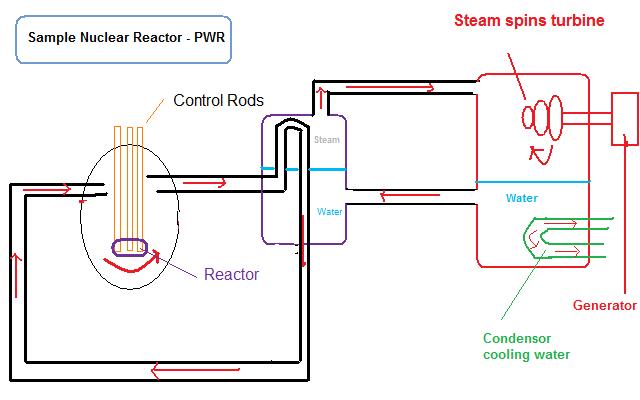
The opposite of a nuclear explosion, nuclear reactors are the controlled release of fission energy. They serve the purpose of converting “nuclear energy” to heat. To produce energy, a nuclear reactor contains several major components: fuel elements (or rods), control rods, and coolant/moderator, besides the vessel itself containing everything. The fuel elements contains the fissile material, typically uranium or plutonium, which is used as the fuel to undergo fission and provide the nuclear energy. The fissile material is encased in a solid cladding, made of Zircalloy (alloy of zirconium, having low capacity to absorb neutrons), to contain both the fuel and the resulting fission products and keep then from escaping into the moderator, coolant, or anywhere outside the cladding. The moderator and coolant flows between the fuel elements (or rods) moderating the neutrons and carrying away the heat. The region inside the nuclear reactor where the fuel elements undergo fission to generate heat is called the nuclear reactor core. The control rods, usually made of cadmium metal, absorb neutrons in order to control the rate of fission. By raising or lowering the control rods in the reactor, the concentration of neutrons, called the neutron flux, in the core increases or decreases respectively. The other component is the coolant. Since the process of fission produces large amounts of heat, the coolant is used to carry away the heat. The heat carried away causes incoming cooler water to turn into steam. This steam spins a turbine, which then powers an electric generator. Generally speaking, water is used, however; helium gas and liquid sodium can be used as substitutes. A pressurized water reactor (PWR) is a common design of a nuclear reactor. In a PWR, water functions as a coolant and as a moderator. A moderator slows down the neutrons, because slower moving neutrons are better at causing fission to occur. A moderator is usually water; however, graphite and heavy water can also be used. A boiling water reactor (BWR) is another common design of current nuclear reactor plants in commercial use. In a BWR, water also functions as both a coolant and moderator. Another type of reactor used in civilian power plants in the former Soviet Union called an RBMK reactor used graphite (carbon) as a moderator, but is considered insufficiently safe.
Most of the power from nuclear fission reactors comes directly from the fission, which can be rapidly stopped by a shutdown of the reactor. However, about 7% comes from heat of decay of highly radioactive fission products, which cannot be stopped by shutting down the reactor, and must continue to be removed from the reactor to prevent overheating and damage to the reactor core. Therefore, coolant pumps must continue to be run for many hours after the reactor is shutdown to remove the decay heat, which over the course of hours eventually decreases. If the coolant pumps do not work, emergency cooling methods must be used to remove decay heat to prevent damage to the core, possible meltdown or release of highly radioactive fission products to where they should not be.
Nuclear Fusion
Nuclear Fusion is the process by which two elements collide to form a new element, releasing a tremendous amount of energy much greater than that of a fission reaction. Similar to nuclear fission, the mass of the resulting element does not exactly match the combined masses of the two smaller elements, but is converted to energy. Stars throughout the universe, including our Sun, release energy through the fusion of two hydrogen atoms into a single helium atom (Figure 3). While electrostatic forces of repulsion would normally drive the positively-charged regions of two hydrogen nuclei away from each other and prevent such a reaction, the extreme heat (15,000,000 °C in the Sun) and density of a star overcomes these forces and allows fusion to occur. With so much potential as a source of energy, the prospect of a fusion reactor on Earth has become a highly sought after technological advancement, even though the challenges of creating such a reactor are immense.
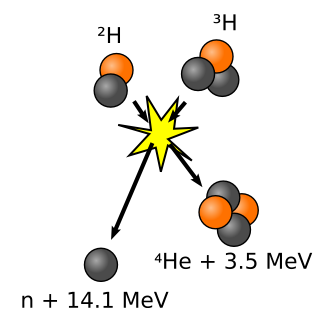
The most promising reaction being attempted by scientists today to produce fusion power is the collision of two hydrogen isotopes, deuterium (2H) and tritium (3H), to produce a 4He atom, a neutron, and a large quantity of energy. Just as in a star, however, the nuclei of the hydrogen isotopes repel each other and must be brought together for fusion at extremely high thermal energies reaching 150,000,000 °C (ten times greater than that required in the Sun). At temperatures this high, the reactant gases transform into a plasma- a hot, fully ionized gas consisting of atomic nuclei and electrons. A problem of central importance to the as of yet theoretical fusion reactor is containing that plasma so that it does not lose thermal energy by touching surrounding materials. A collaborative effort funded by multiple nations known as the International Thermonuclear Experimental Reactor (ITER) aims to solve this problem by confining the plasma in a magnetic field created by powerful superconducting magnets. Such a design is known as a tokamak reactor (See Figure 4).

While the feasibility of a controlled fusion reaction occurring on Earth has yet to be adequately verified, the potential benefits of fusion as opposed to fission may be immense. Deuterium may be extracted from water and lithium, the tritium source for the fusion reaction, is estimated to exist on earth in quantities that will last for one million years. Additionally, there is far less nuclear waste that decays much faster compared to that produced by fission.
Nuclear Safety
There have been three major accidents involving full-scale civilian nuclear power plants.
- The first occurred in 1979 at Three Mile Island Unit 2 in Pennsylvania. Due mechanical failure, the main water pumps stopped running, leading to a partial meltdown of the fuel rods. Excessive heat caused a fracture in one of the reactors, allowing a small amount of radioactive steam into the atmosphere. Fortunately, no one was killed or even injured. This incident also lead to heightened regulation and safety precautions of nuclear reactors in the United States.
- On April 26, 1986, the worst accident in nuclear history occurred in Chernobyl, Ukraine. During a routine test, an uncontrollable power surge burned the control rods, and massive amounts of radioactive smoke were released. 237 people suffered from acute radiation sickness, and 31 died within the first three months of the accident. Other effects of the radiation included an increase in down's syndrome, chromosomal aberrations, neural tube defects, and thyroid cancer. Perhaps the most important effect was psychological as the accident caused severe anxiety for the survivors and a general lack of trust in the government.

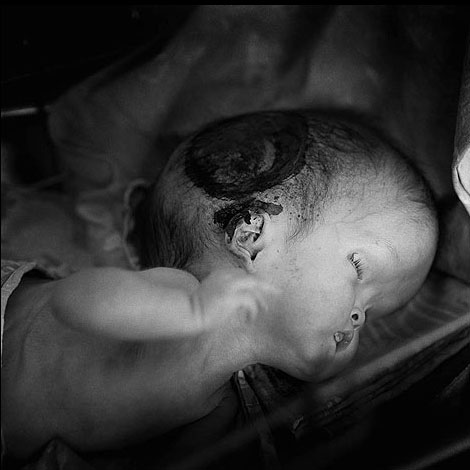
- Due to a severe earthquake and tsunami in Japan in March 11, 2011, several BWR (Boiling Water Reactor) nuclear reactors at the Fukushima power plant lost electrical power for cooling, underwent explosions, and suffered reactor core damage from post-shutdown decay heat coming from highly radioactive fission products. Workers eventually pumped seawater into the reactors to cool them down and limit any further damage.
Problems
- What is the function of control rods in a nuclear reactor?
- True or False? The accident at Three Mile Island led to the radiation poisoning of possibly hundreds of thousands of people.
- How does nuclear fission lead to a chain reaction?
- What two functions can water serve in a nuclear reactor?
- What is critical mass? What will occur if the mass of a reaction surpasses its critical mass?
Answers
- In a fission reactor, control rods absorb neutrons to control the rate of a reaction. Lowering the rods into the reactor decreases the rate of fission and removing them increases the rate.
- False. The accident at Three Mile Island was only minor and no one was killed or injured.
- When a neutron strikes a fissile material breaking it into smaller fragments, more neutrons are released (2.5 on average). These neutrons then collide with other fissile atoms producing even more neutrons, which continue the chain reaction either in a stable manner (reactor) or violently (atomic bomb).
- In a fission reactor, water serves as a moderator (slows down highly energetic neutrons to the appropriate thermal energy for a reaction) and produces steam by coming into contact with the hot water near the reactor and transferring heat. This steam can then power turbines to generate electricity.
- The critical mass of a fission reactor is the mass of fissile material required to maintain a chain reaction. If the mass of a reaction surpasses its critical mass, the result is an uncontrolled chain reaction that culminates in a large explosion (this is how an atomic bomb works).
External Links
For more information on the subject of nuclear reactor, the following links may be helpful:
- http://en.Wikipedia.org/wiki/Nuclear_Reactor_Technology
- http://www.howstuffworks.com/nuclear-power.htm
- http://www.youtube.com/watch?v=rvAJ_u3Q0Hw&feature=related
- people.moreheadstate.edu/students/alsimp01 /files/technology.html
References
- Petrucci, Ralph H., et al. “Nuclear Reactors.” General Chemistry: Principles & Modern Applications. New Jersey: Pearson Education, Inc, 2007. 1057-1058.
- Bloomfield, Louis A. How Things Work: The Physics of Everyday Life, Second Edition. New York: John Wiley&Sons Inc, 2001. 445.
- AJ Software & Multimedia. Nuclear Fission: Basics. 1998-2008. <http://www.atomicarchive.com/Fission/Fission1.shtml>. United States Nuclear Regulatory Commission. Backgrounder on the Three Mile Island Accident. 11 August 2009. 29 November 2009 <www.nrc.gov/reading-rm/doc-co...mile-isle.html>.
- World Nuclear Association. Chernobyl Accident. November 2009. 2009 November 2009 <www.world-nuclear.org/info/ch...byl/inf07.html>.
- International Thermonuclear Experimental Reactor (ITER). "What is Fusion?" November 22 2010. Web. 26 May 2011.
- Nuclear Power Education. "Everything You Want to Know about Nuclear Power." September 3 2010. Web. 26 May 2011.
Contributors and Attributions
- Anna Becker (UCD)

