Acid and Base Indicators
- Page ID
- 97047
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The most common method to get an idea about the pH of solution is to use an acid base indicator. An indicator is a large organic molecule that works somewhat like a " color dye". Whereas most dyes do not change color with the amount of acid or base present, there are many molecules, known as acid - base indicators , which do respond to a change in the hydrogen ion concentration. Most of the indicators are themselves weak acids.
Indicators
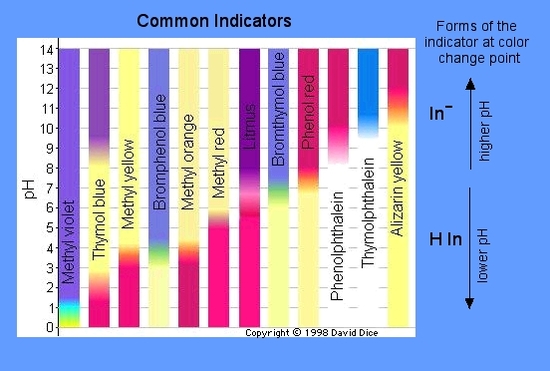
The most common indicator is found on "litmus" paper. It is red below pH 4.5 and blue above pH 8.2.
| Color | Blue Litmus | Red Litmus |
|---|---|---|
| Acid | turns red | stays same |
| Base | stays same | turns blue |
Other commercial pH papers are able to give colors for every main pH unit. Universal Indicator, which is a solution of a mixture of indicators is able to also provide a full range of colors for the pH scale.
A variety of indicators change color at various pH levels. A properly selected acid-base indicator can be used to visually "indicate" the approximate pH of a sample. An indicator is usually some weak organic acid or base dye that changes colors at definite pH values. The weak acid form (HIn) will have one color and the weak acid negative ion (In-) will have a different color. The weak acid equilibrium is:
HIn → H+ + In-
- For phenolphthalein: pH 8.2 = colorless; pH 10 = red
- For bromophenol blue: pH 3 = yellow; pH 4.6 = blue
See the graphic below for colors and pH ranges.
Magic Pitcher Demonstration
Phenolphthalein is an indicator of acids (colorless) and bases (pink). Sodium hydroxide is a base, and it was in the pitcher at the beginning, so when added to the phenolphthalein in beakers 2 and 4, it turned pink (top half of the graphic).
Reaction
Equilibrium: HIn → H+ + In-
colorless red
The equilibrium shifts right, HIn decreases, and In- increases. As the pH increase between 8.2 to 10.0 the color becomes red because of the equilibrium shifts to form mostly In- ions.
- The third beaker has only the NaOH but no phenolphthalein, so it remained colorless. The first beaker contain acetic acid and is skipped over at first.
- After pouring beakers 2, 3, 4 back into the pitcher it give a pink solution.
- Bottom half of the graphic: When the pitcher is then poured back into beakers 2, 3, 4 it is a pink solution.
- In the first beaker, a strange thing happens in that the pink solution coming out of the pitcher now changes to colorless. This happens because the first beaker contains some vinegar or acetic acid which neutralizes the NaOH, and changes the solution from basic to acidic. Under acidic conditions, the phenolphthalein indicator is colorless.
Neutralization reaction:
HC2H3O2 + NaOH → Na(C2H3O2) + HOH
Explain the color indicator change
Use equilibrium principles to explain the color change for phenolphthalein at the end of the demonstration.
Solution
The simplified reaction is: H+ + OH- → HOH
As OH- ions are added, they are consumed by the excess of acid already in the beaker as expressed in the above equation. The hydroxide ions keep decreasing and the hydrogen ions increase, pH decreases.
See lower equation: The indicator equilibrium shifts left, In- ions decrease. Below pH 8.2 the indicator is colorless. As H+ ions are further increased and pH decreases to pH 4-5, the indicator equilibrium is effected and changes to the colorless HIn form.
Equilibrium: HIn → H+ + In-
colorless red

Molecular Basis for the Indicator Color Change
Color changes in molecules can be caused by changes in electron confinement. More confinement makes the light absorbed more blue, and less makes it more red.
How are electrons confined in phenolphthalein? There are three benzene rings in the molecule. Every atom involved in a double bond has a p orbital which can overlap side-to-side with similar atoms next to it. The overlap creates a 'pi bond' which allows the electrons in the p orbital to be found on either bonded atom. These electrons can spread like a cloud over any region of the molecule that is flat and has alternating double and single bonds. Each of the benzene rings is such a system.
See the far left graphic - The carbon atom at the center (adjacent to the yellow circled red oxygen atom) doesn't have a p-orbital available for pi-bonding, and it confines the pi electrons to the rings. The molecule absorbs in the ultraviolet, and this form of phenolphthalein is colorless.
In basic solution, the molecule loses one hydrogen ion. Almost instantly, the five-sided ring in the center opens and the electronic structure around the center carbon changes (yellow circled atoms) to a double bond which now does contain pi electrons. The pi electrons are no longer confined separately to the three benzene rings, but because of the change in geometry around the yellow circled atoms, the whole molecule is now flat and electrons are free to move within the entire molecule. The result of all of these changes is the change in color to pink. Chime: Phenolphthalein
Many other indicators behave on the molecular level in a similar fashion (the details may be different) but the result is a change in electronic structure along with the removal of a hydrogen ion from the molecule. Plant pigments in flowers and leaves also behave in this fashion.

