1.87: Planck's Radiation Equation Fit to Experimental Data
- Page ID
- 157655
\[
\mathrm{n} :=42 \qquad \mathrm{i} :=1 . . \mathrm{n}
\nonumber \]
| \(\rho_{i} :=\) | \(\lambda_{i} :=\) |
| 0.07 | 0.667 |
| 0.096 | 00.720 |
| 0.10 | 0.737 |
| 0.190 | 0.811 |
| 0.210 | 0.383 |
| 0.398 | 0.917 |
| 0.420 | 0.917 |
| 0.680 | 1.027 |
| 0.708 | 1.021 |
| 1.036 | 1.167 |
| 1.062 | 1.172 |
| 1.258 | 1.247 |
| 1.669 | 1.484 |
| 1.770 | 1.697 |
| 1.776 | 1.831 |
| 1.730 | 2.039 |
| 1.685 | 2.170 |
| 1.640 | 2.275 |
| 1.551 | 2.406 |
| 1.392 | 2.563 |
| 1.145 | 2.27 |
| 1.115 | 2.824 |
| 1.071 | 2.916 |
| 1.042 | 2.921 |
| 0.974 | 3.050 |
| 0.918 | 2.151 |
| 0.797 | 3.344 |
| 0.760 | 3.450 |
| 0.742 | 3.556 |
| 0.698 | 3.661 |
| 0.667 | 3.754 |
| 0.570 | 4.027 |
| 0.426 | 4.427 |
| 0.378 | 4.613 |
| 0.345 | 4.805 |
| 0.310 | 4.968 |
| 0.280 | 5.128 |
| 0.250 | 5.296 |
| 0.220 | 5.469 |
| 0.205 | 5.632 |
| 0.175 | 5.783 |
| 0.155 | 6.168 |
The data for this exercise is taken from page 19 of Eisberg and Resnick, Quantum Physics.
The values of rho are given in units of 103 joules/m3 and the values of lambda are given in 10‐6 m. The temperature is 1595 K.
Two pairs of data points are used to get approximate values for the parameters a and b in the Planck equation.
\[
a :=1 \qquad \mathrm{b} :=1
\nonumber \]
Given
\[
\rho_{16}=\frac{a \cdot\left(\lambda_{16}\right)^{-5}}{e^{\frac{b}{\lambda_{16}}}} \qquad \rho_{22}=\frac{a \cdot\left(\lambda_{22}\right)^{-5}}{e^{\frac{b}{\lambda_{22}}}-1}
\nonumber \]
\[
\left(\begin{array}{l}{\mathrm{a}} \\ {\mathrm{b}}\end{array}\right) :=\text { Find }(\mathrm{a}, \mathrm{b}) \qquad \mathrm{a}=3.84 \times 10^{3} \qquad \mathrm{b}=8.479
\nonumber \]
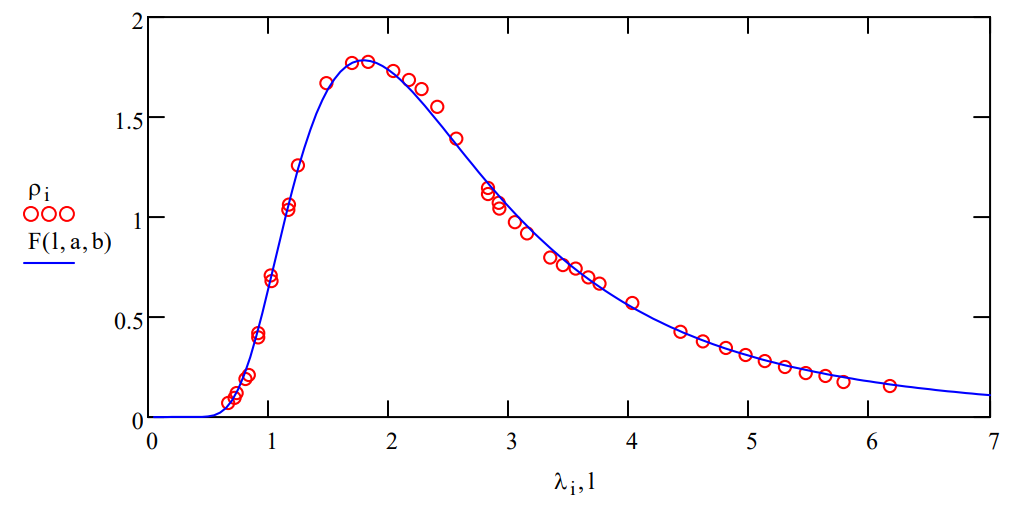
Planck's Equation is fit to data:
\[
\mathrm{F}(\lambda, \mathrm{a}, \mathrm{b}) :=\frac{\mathrm{a} \cdot \lambda^{-5}}{e^{\frac{\mathrm{b}}{\lambda}}-1}
\nonumber \]
\[
\operatorname{SSD}(\mathrm{a}, \mathrm{b}) :=\sum_{\mathrm{i}}\left(\rho_{\mathrm{i}}-\mathrm{F}\left(\lambda_{\mathrm{i}}, \mathrm{a}, \mathrm{b}\right)\right)^{2}
\nonumber \]
Given
\[
\operatorname{SSD}(\mathrm{a}, \mathrm{b})=0 \qquad \mathrm{a}>0 \qquad \mathrm{b}>0 \qquad\left(\begin{array}{l}{\mathrm{a}} \\ {\mathrm{b}}\end{array}\right) :=\operatorname{Minerr}(\mathrm{a}, \mathrm{b})
\nonumber \]
Display optimum values of a and b:
\[
\mathrm{a}=4.715 \times 10^{3} \qquad \mathrm{b}=8.906
\nonumber \]
Plot of fit:
\[
1 :=0.05, 0.1 \ldots 7
\nonumber \]
Calculate Planck's constant using the value of b, which is equal to (hc)/(kT).
\[
\mathrm{h} :=\frac{\mathrm{b} \cdot 10^{-6} \cdot 1.381 \cdot 10^{-23} \cdot 1595}{2.9979 \cdot 10^{8}} \qquad \mathrm{h}=6.544 \times 10^{-34}
\nonumber \]

