3.E: The Schrödinger Equation and a Particle in a Box (Exercises)
- Page ID
- 13402
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Solutions to select questions can be found online.
3.2
Determine from the following operators which are linear and nonlinear:
- \(\hat{A}f(x)= f(x)^2\) [square f(x)]
- \(\hat{A}f(x)= f^*(x)\) [form the complex conjugate of f(x)]
- \(\hat{A}f(x)= 0\) [multiply f(x) by zero]
- \(\hat{A}f(x)= [f(x)]^{-1}\) [take the reciprocal of f(x)]
- \(\hat{A}f(x)= f(0)\) [evaluate f(x) at x=0]
- \(\hat{A}f(x)= \ln f(x)\) [take the log of f(x)]
- Solution
-
It is important to note that an operator \(\hat{A}\) is linear if
\[ \underbrace{\hat{A}[c_1f(x)+c_2f_2(x)]}_{\text{left side}}= \underbrace{c_1\hat{A}f_1(x)+c_2\hat{A}f_2(x) }_{\text{right side}}\nonumber \]
and the operator is nonlinear if
\[ \underbrace{ \hat{A}[c_1f_1(x)+c_2f_2(x)]}_{\text{left side}} \neq \underbrace{ c_1\hat{A}f_1(x)+c_2\hat{A}f_2(x) }_{\text{right side}}\nonumber \]
a)
Evaluate the left side
\[\begin{align*} \hat{A}[c_1f(x)+c_2f_2(x)] &= [c_1f_1(x)+c_2f_2(x)]^2 \\ &= c_1^2 f_1(x)^2+2c_1f_1(x) c_2f_2(x)+c_2^2f_2(x)^2 \end{align*}\nonumber \]
Evaluate the right side
\[c_1 \hat{A} f_1(x)+c_2\hat{A}f_2(x)=c_1[f_1(x)]^2+c_2[f_2(x)]^2 \neq \hat{A}[c_1f_1(x)+c_2f_2(x)] \nonumber \]
This operator is nonlinear
b)
Evaluate the left side
\[\hat{A}[c_1f_1(x)+c_2f_2(x)] = c_1^*f_1^*(x) + c_2^*f_2^*(x)\nonumber \]
Evaluate the right side
\[\begin{align*} c_1\hat{A}f_1(x) + c_2\hat{A}f_2(x) &= c_1f_1^*(x) + c_2f_2^*(x) \\[4pt] &= \hat{A}[c_1f_1(x) + c_2f_2(x)] \end{align*} \]
This operator is linear
c)
Evaluate the left side
\[ \hat{A}[c_1f_1(x)+c_2f_2(x)] = 0\nonumber \]
Evaluate the right side
\[c_1\hat{A}f_1(x) + c_2\hat{A}f_2(x) = c_1f_1(x) + c_2f_2(x) = 0\nonumber \]
\[ = \hat{A}[c_1f_1(x) + c_2f_2(x)]\nonumber \]
This operator is linear
d)
Evaluate the left side
\[\hat{A}[c_1f_1(x)+c_2f_2(x)] = \dfrac{1}{c_1f_1(x) + c_2f_2(x)}\nonumber \]
Evaluate the right side
\[c_1\hat{A}f_1(x) + c_2\hat{A}f_2(x) = \dfrac{c_1}{f_1(x)} + \dfrac{c_2}{f_2(x)} \nonumber \]
\[ \neq \hat{A}[c_1f_1(x) + c_2f_2(x)]\nonumber \]
This operator is nonlinear
e)
Evaluate the left side
\[\hat{A}[c_1f_1(x)+c_2f_2(x)] = c_1f_1(0) + c_2f_2(0)\nonumber \]
Evaluate the right side
\[ = c_1\hat{A}f_1(x) + c_2\hat{A}f_2(x)\nonumber \]
This operator is linear
f)
Evaluate the left side
\[\hat{A}[c_1f_1(x)+c_2f_2(x)] = \ln [c_1f_1(x) + c_2f_2(x)]\nonumber \]
Evaluate the right side
\[c_1\hat{A}f_1(x) + c_2\hat{A}f_2(x) = c1 \ln f_1(x) + c_2 \ln f_2(x)\nonumber \]
\[ \neq \hat{A}[c_1f_1(x) + c_2f_2(x)]\nonumber \]
This operator is nonlinear
3.8
Show that for a particle in a box with length a with state \(n=3\) that there are 3 locations along the x axis where the probability density is at a maximum.
- Solution
-
The probability density for a particle in a box for state \(n=3\) is
\[{\psi}^*{\psi}=\dfrac{2}{a}{\sin}^2\dfrac{3px}{a}\nonumber \]
To maximize the probability density, take its derivative and set it equal to zero and solve for \(x\) .
\[\dfrac{d}{dx}\left[\dfrac{2}{a}{{\sin}^2 \dfrac{3{\pi}x}{a}\ }\right]=\dfrac{2}{a}\cdot 2\cdot {\sin \dfrac{3{\pi}x}{a}\ }\cdot {\cos \dfrac{3{\pi}x}{a}\ }\cdot \dfrac{3\pi}{a}=0\nonumber \]
\[{\sin \dfrac{3{\pi}x}{a}\ }{\cos \dfrac{3{\pi}x}{a}\ }=0\nonumber \]
We want to not choose values of x that make \({\sin \dfrac{3{\pi}x}{a}\ }=0\) , as that means that the probability density will be zero. We will only choose the zeros of \({\cos \dfrac{3{\pi}x}{a}\ }\). So the possible values for x which make
\[{\cos \dfrac{3{\pi}x}{a}\ } =0 \nonumber \]
are
\[\dfrac{3px}{a}=\dfrac{2m+1}{2}p\ \ \ \ \ \ m=0,1,2,\dots \nonumber \]
\[x=\dfrac{\left(2m+1\right)a}{6}\nonumber \]
We only choose \(m=0,1,2\) and not 3 because \(m=3\) would give \(x=\dfrac{7a}{6}\) which is outside the box. So the locations are
\[x=\dfrac{a}{6}\nonumber \]
\[x=\dfrac{a}{2}\nonumber \]
\[x=\dfrac{5a}{6}\nonumber \]
3.13
What range for \(L\) is possible for \(\sigma_x\) given:
\[\sigma_x = \sqrt{\langle x^2\rangle - \langle x \rangle^2}\nonumber \]
where \(L\) is the length of the 1-D box? Hint: Remember that \(\sigma_x\) is the uncertainty in the position of a particle in a box.
- Solution
-
For a particle in a box:
\(\langle x \rangle = \dfrac{\text{L}}{2}\)
and
\(\langle x^2 \rangle = \dfrac{L^2}{3}-\dfrac{L^2}{2n^2\pi^2}\)
\(\sigma_x = \sqrt{\dfrac{L^2}{3}-\dfrac{L^2}{2n^2\pi^2} - (\dfrac{\text{L}}{2})^2}\)
By inspection, only values of \(\sigma_x\) less than \(L\) will make this statement true.
3.14
Using the trigonometric identity
\[\cos(2x)=2\cos^{2}x-1\nonumber \]
show that
\[\int_0^a 2 \cos^2 \dfrac{n\pi x}{a} -1 dx = 0\nonumber \]
\[\int_0^a \cos \dfrac{2n\pi x}{a} dx = 0\nonumber \]
\[\dfrac{a}{2n\pi} \sin(2n\pi) = 0\nonumber \]
3.18
Is the wavefunction \( \phi_n= \sqrt{\dfrac{2}{L}} \sin{(\dfrac{n\pi x}{L})} \) orthonormal over \( 0 \leq{x} \leq{L}\). Explain your reasoning.
- Solution
-
For a wavefunction to be orthonormal, it has to satisfy these conditions 1.) it has to be orthogonal and 2.) it has to be normalized.
To show that it is orthogonal:
\[ \int_0^L \phi_m \phi\ast_n dx = 0\nonumber \]
when \(m\neq\) n
To show that that the wavefunction is normalized it must follow that
\[ \int_0^L \phi_n \phi\ast_n dx = 1 \nonumber \]
when m=n
Because our wavefunction satisfies both conditions, it is an orthonormal function.
We find that
\[\langle \psi_3 |\psi_3 \rangle = \int \limits _{-\infty}^{\infty} \dfrac{2}{L}(\sin\dfrac{3\pi x}{L})^2 dx = 1\nonumber \]
and
\[\langle \psi_4 |\psi_4 \rangle = \int \limits _{-\infty}^{\infty} \dfrac{2}{L}(\sin\dfrac{3\pi x}{L})(\sin\dfrac{4\pi x}{L}) dx = 0\nonumber \]
From orthogonality, we can learn that if n is not equal to m, our dot product will always be zero. But if n = m our dot product will equal 1.
3.22
What is the Heisenberg Uncertainty Principle? Do position and momentum follow the uncertainty principle; why or why not? If they do, what is the minimum uncertainty in the velocity of an electron if it is known to be within 1.5nm of a nucleus?
- Solution
-
The Heisenberg Uncertainty Principle states that two properties that follow cannot be simultaneously measured to arbitrary precision. Position and momentum follow the principle. If one were to try and commute these two operators, one would not get zero and therefore the properties do not commute. If they do not commute then they cannot be measured to arbitrary precision.
We know that
\[\Delta x \Delta p \geq \dfrac{\hbar}{2}\nonumber \]
And that p = mv. This gives
\[m\Delta x \Delta v \geq \dfrac{\hbar}{2}\nonumber \]
The mass of an electron is known is \(m_e \approx 9.1 \times 10^{-31}\;kg \). The problem also gives \(\Delta x\) to be 1.5 nm. From here, it becomes a plug and chug to solve for \(\Delta v\).
\[\Delta v = 3.86 \times 10^4\nonumber \]
3.23
Describe the degeneracies of a two-dimensional box whose two sides have different lengths.
- Solution
-
The energies of a two-dimensional box is given by,
\[E = \dfrac{h^2}{8m} \left(\dfrac{n^{2}_{x}}{a^2}+\dfrac{n^{2}_{y}}{b^2}\right)\nonumber \]
We can see that even if \(a \ne b\), the energy levels will not necessarily be degenerate.
3.26
How many degenerate states do the first three energy levels for a three-dimensional particle in a box have if \(a=b=c\)?
3.27
Metal porphyrin molecules are commonly in many proteins and it has the general structure.
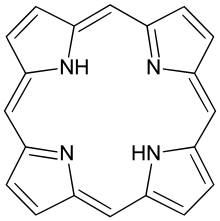
This molecule is planar, so we can approximate π electrons as being confined inside a square. What are energy levels and corresponding degeneracies of a particle in a square of side \(m\)? Porphyrin molecules have 18 \(π\) electrons. If the length of the molecule is 850 pm, what is the lowest energy absorption of the porphyrin molecules? (the experimental value ≈ 17,000 cm-1)
- Solution
-
The first energy level is E(1,1,1) which has no degeneracy.
The second energy state is E(2,1,1)=E(1,2,1)=E(1,1,2), therefore it has three degenerate states.
The third energy state is E(2,2,1)=E(2,1,2)=E(1,2,2), therfeore it has three degenerate states.
3.26
For a two dimensional box of width \(w\) and height \(h=\sqrt{a}w\), calculate all possible energy combinations between \(E_{11}\) and \(E_{33}\) note any degeneracy.
- Solution
-
The energy of a two dimensional particle in the box has the form,
\[E = \dfrac{h^2}{8m}\Bigg(\dfrac{n_x^2}{w^2}+\dfrac{n_y^2}{h^2}\Bigg)\nonumber \]
In this specific case \(h=\sqrt{a}w\) so we can simplify the problem to,
\[E = \dfrac{h^2}{8m}\Bigg(\dfrac{n_x^2}{w^2}+\dfrac{2n_y^2}{w^2}\Bigg)\nonumber \]
Now we can tabulate the energy level indicating degeneracy.
\(E_{xy}\) Degeneracy \(\dfrac{E8mw^2}{h^2}\) \(E_{11}\) 1 3 \(E_{12}\). \(E_{31}\) 2 5 \(E_{21}\) 1 4 \(E_{22}\) 1 6 \(E_{13}\). \(E_{32}\) 2 7 \(E_{23}\) 1 8 \(E_{33}\) 1 9
3.32
In this problem, we will explore the quantum-mechanical problem of a free particle that is not restricted to a finite region. Remember quantized energies of a particle in a box is a direct result from the boundary conditions set by the confines of the box.
When the potential energy \(V(x)\) is equal to zero and the Schrodinger equation become
\[\dfrac{d^2\psi}{dx^2} + \dfrac{2mE}{\hbar^2}\psi(x) = 0\nonumber \]
The two solutions to this Schrodinger equation are
\[\psi_1(x) = A_1e^{ikx}\nonumber \]
\[\psi_2(x) = A_2e^{-ikx}\nonumber \]
Show that \(\psi_1(x)\) and \(\psi_2(x)\) are solution to the Schrodinger equation where the potential energy \(V(x)\) is equal to zero
- Solution
-
In order to prove that \(\psi_1(x)\) and \(\psi_2(x)\) are solutions we need to mention a few values
\[p = \hbar k → k = p/\hbar →k = \dfrac{(2mE)^{1/2}}{\hbar}\nonumber \]
Now we have
\[\dfrac{d^2Ae^{\pm ikx}}{dx^2} + \dfrac{2mE}{\hbar^2}Ae^{\pm ikx}= 0\nonumber \]
\[A(\pm ik)^2e^{\pm ikx} + \dfrac{2mE}{\hbar^2}Ae^{\pm ikx} = 0 → -k^2 + \dfrac{2mE}{\hbar^2} = 0 \nonumber \]
Cancel the like terms
Thus, \(k = \dfrac{(2mE)^{1/2}}{\hbar}\), which equals the original \(k\) value
3.32
Show that \(E\) had to be a positive value, since when \(E\) is negative the wave function become unbounded for large \(x\) values
- Solution
-
If \(E\) < 0 then k becomes imaginary, \(k = ik\)
\(\psi = Ae^{\pm ikx} = Ae^{\pm i(ik)x} Ae^{\pm kx} \)
For \(\psi_1(x) = A_1e^{-kx} \) this will blow up for x → - \(\infty\)
For \(\psi_2(x) = A_2e^{kx} \) this will blow up for x → \(\infty\)
3.32
With \(\hat{P}\) \(\psi_1(x)\) and \(\hat{P}\) \(\psi_2(x)\) as eigenvalue equations, show that
\[\hat{P}\psi_1(x) = -i\hbar \dfrac{d\psi_1}{dx} = \hbar k\psi_1\nonumber \]
and
\[\hat{P}\psi_2(x) = -i\hbar \dfrac{d\psi_2}{dx} = -\hbar k\psi_2\nonumber \]
- Solution
-
\[\hat{P}\psi_1(x) = -i\hbar \dfrac{d\psi_1}{dx} = -i\hbar \dfrac{d}{dx}A_1e^{+ikx} = -i\hbar A_1(ik)e^{ikx} = +\hbar kA_1e^{ikx} = + \hbar k\psi_1\nonumber \]
\[\hat{P}\psi_2(x) = -i\hbar \dfrac{d\psi_2}{dx} = -i\hbar \dfrac{d}{dx}A_2e^{-ikx} = -i\hbar A_2(-ik)e^{ikx} = -\hbar kA_1e^{-ikx} = -\hbar k\psi_2\nonumber \]
Now we can show that
\[E = \dfrac{p^2}{2m} = \dfrac{\pm (\hbar)^2}{2m} = \dfrac{\hbar^2 k^2}{2m}\nonumber \]
3.32
Show that \(\psi_1^{*}\psi_1(x) = A_1^{*}A_1 = \left | A_1\right |^2\) and that \(\psi_2^{*}\psi_2(x) = A_2^{*}A_2 = \left | A_2\right |^2\)
- Solution
-
\[\begin{align*} \psi_1^{*}\psi_1(x) &= (A_1e^{ikx})^*A_1e^{ikx} \\[4pt] &= A_1^{*}A_1e^{-ikx}e^{ikx} \\[4pt] &= A_1^{*}A_1e^{-ikx+ikx} = A_1^{*}A_1e^{0} \\[4pt] &= A_1^{*}A_1\end{align*} \]
\[\begin{align*} \psi_2^{*}\psi_2(x) &= (A_2e^{-ikx})^*A_2e^{-ikx} \\[4pt] &= A_2^{*}A_2e^{ikx -ikx} \\[4pt] &= A_2^{*}A_2e^{0} = A_2^{*}A_2 \end{align*} \]
\(\psi\) has equal probability to be everywhere when \(\Delta x = \infty\) and \(\Delta p = 0 \)
3.33A
Assuming that a particle is characterized by a standing de Broglie wave, come up with an equation for the allowed energies of a particle in a one-dimensional box.
- Solution
-
The de Broglie relationship is
\[\lambda = \dfrac{h}{p}\nonumber \]
Because the waves are standing waves, an integral number of half wave-lengths will fit in the box or:
\[a = \dfrac{n\lambda}{2}\nonumber \]
and
\[a = \dfrac{nh}{2p}\nonumber \]
Solving for \(p\) yields
\[p = \dfrac{nh}{2a}\nonumber \]
and the corresponding energy is
\[E = \dfrac {mv^2}{2} = \dfrac{p^2}{2m} =\dfrac{1}{2m} \dfrac {n^2h^2}{4a^2} = \dfrac{n^2h^2}{8ma^2} \nonumber \]
3.33B
Derive the lowest allowed velocity for a proton in a box of length 10-14 m (approximate size of nucleus), assuming the particle is described by a standing deBroglie wave.
- Solution
-
The de Broglie wavelength is
\[λ = \dfrac{h}{p} = \dfrac{h}{m_pv}\nonumber \]
For a one dimensional wave that has nodes on both ends of a box, an integer number of half wavelengths can fit, so
\[n \left(\dfrac{λ}{2} \right) = L\nonumber \]
Substituting this wavelength in the de Broglie relationship, one gets
\[\nu = \dfrac{hn}{2m_L}\nonumber \]
lowest allowed velocity will have \(n = 1\)
\[\begin{align*} \nu &= \dfrac{(6.626 \times 10^{-34})(1)}{2 \times (1.67 \times 10^{-27})(10^{-14})} \\[4pt] &= 19.8 \times 10^6 m/s \end{align*} \]
3.33C
If a particle in a one-dimensional box is described by standing de Broglie waves within the box, derive an equation for the allowed energies. Then use that equation to find the transition energy from n=1 to n=2 given the length of the box is 350 pm and the mass of an electron is \(9.109 \times 10^{-31} kg\).
- Solution
-
The de Broglie formula is
\[\lambda=\dfrac{h}{p}\nonumber \]
An integral number of half-wavelengths will fit in the box because the waves are standing waves so
\[\dfrac{n\lambda}{2}=a\nonumber \]
\[\dfrac{nh}{2p}=a\nonumber \]
Then solving for p
\[p=\dfrac{nh}{2a}\nonumber \]
Therefore the energy equation is
\[E=\dfrac{mv^2}{2}=\dfrac{p^2}{2,m}=\dfrac{1}{2m} \dfrac{n^2h^2}{4a^2}=\dfrac{n^2h^2}{8ma^2}\nonumber \]
Just plug into the equation to find the transition energy
\[\Delta E=\dfrac{h^2}{8m_ea^2}(2^2-1^2)\nonumber \]
\[\Delta E=\dfrac{(6.626 \times 10^{-34} J \centerdot s)^2 (3) }{8(9.109 \times 10^{-31} kg)(350 \times 10^{-12}m)^2}\nonumber \]
\[\Delta E=1.47 \times 10^{-18}J \nonumber \]
3.35A
Consider the two wavefunctions
\[\psi_n(x) = \sin\dfrac{n\pi x}{a} \nonumber \]
with even n numbers and
\[\psi_n(x) = \cos\dfrac{n\pi x}{a} \nonumber\]
with odd n numbers.
Prove that the wavefunctions can be symmetric and antisymmetric by using the operation x to -x, a is a constant.
Given that the Schrödinger equation has the expression:
\[\hat{H}(x)\psi_n(x) = E_n\psi_n(x) \nonumber\]
Through the operation x to -x, the equation now becomes:
\[\hat{H}(-x)\psi_n(-x) = E_n\psi_n(-x) \nonumber\]
Show that
\[\hat{H}(x) = \hat{H}(-x)\nonumber\]
is true to prove the Schrödinger equation.
- Solution
-
Substituting \(x\) by \(-x\), for odd \(n\) numbers,
\[\hat{\psi}_n(-x) = \cos\dfrac{-n\pi x}{a} = \cos\dfrac{n\pi x}{a} = \hat{\psi}_n(x) \]
For even \(n\) numbers,
\[\hat{\psi}_n(-x) = sin\dfrac{-n\pi x}{a} = -sin\dfrac{n\pi x}{a} = -\hat{\psi}_n(x) \]
Thus, the wavefunction for odd \(n\) number is symmetric and even n numbers is antisymmetric.
And,
\[\hat{H}(x) = -\dfrac{\hbar^2}{2m}\dfrac{d^2}{dx^2} = \hat{H}(x)\]
\[\hat{H}(-x) = -\dfrac{\hbar^2}{2m}\dfrac{d^2}{d(-x)^2} = \hat{H}(x)\]
Thus,
\[\hat{H}(x) = \hat{H}(-x) \nonumber\]
and
\[\hat{H}(x) \nonumber \]
is an even function of \(x\).
3.35B
Show that the Hamiltonian for a Rigid Rotor Model is odd.
- Solution
-
\[ \hat{H}(x) = \hat{H}(-x)\nonumber \]
so
\[\hat{H}= -\dfrac{h^2}{4\pi \mu}\nabla^2\nonumber \]
\[\nabla^2 = \dfrac{d^2y}{dx^2} \dfrac{d^2y}{dy^2}\dfrac{d^2y}{dz^2}\nonumber \]
so
\[\dfrac{d^2y}{dx^2} (x) = 0\nonumber \]
and
\[\dfrac{d^2y}{dx^2}(-x) = 0\nonumber \]
so
\[\hat{H}(x) = 0\nonumber \]
and
\[\hat{H}(-x) = 0\nonumber \]
so
\[\hat{H}(x)= \hat{H}(-x)\nonumber \]

