2.1: Boyle's Law
- Page ID
- 151823
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
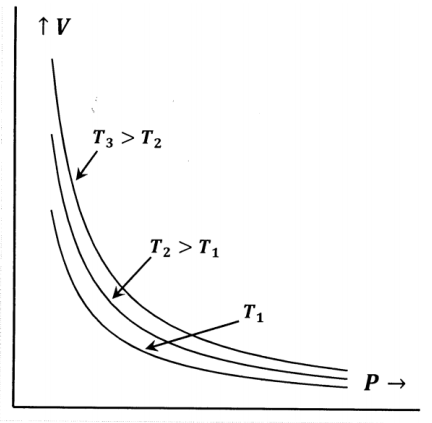
Robert Boyle discovered Boyle’s law in 1662. Boyle’s discovery was that the pressure, P, and volume, V, of a gas are inversely proportional to one another if the temperature, T, is held constant. We can imagine rediscovering Boyle’s law by trapping a sample of gas in a tube and then measuring its volume as we change the pressure. We would observe behavior like that in Figure 1. We can represent this behavior mathematically as
\[PV={\alpha }^*(n,T) \nonumber \]
where we recognize that the “constant”, \({\alpha }^*\), is actually a function of the temperature and of the number of moles, \(n\), of gas in the sample. That is, the product of pressure and volume is constant for a fixed quantity of gas at a fixed temperature.
A little thought convinces us that we can be more specific about the dependence on the quantity of gas. Suppose that we have a volume of gas at a fixed pressure and temperature, and imagine that we introduce a very thin barrier that divides the volume into exactly equal halves, without changing anything else. In this case, the pressure and temperature of the gas in each of the new containers will be the same as they were originally. But the volume is half as great, and the number of moles in each of the half-size containers must also be half of the original number. That is, the pressure–volume product must be directly proportional to the number of moles of gas in the sample:
\[PV=n\alpha (T) \nonumber \]
where \(\alpha (T)\) is now a function only of temperature. When we repeat this experiment using different gaseous substances, we discover a further remarkable fact: Not only do they all obey Boyle’s law, but also the value of \(\alpha (T)\) is the same for any gas.