Electrophilic Attack on Conjugated Dienes-Kinetic and Thermodynamic Control
- Page ID
- 55208
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
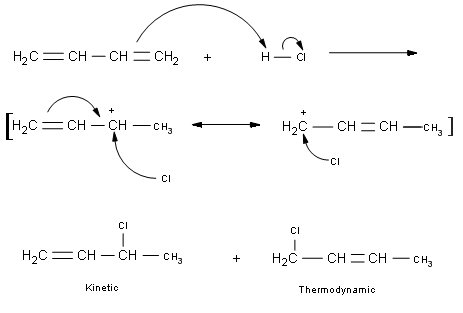
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)When a conjugated diene is attacked by an electrophile, the resulting products are a mixture of 1,2 and 1,4 isomers. Kinetics and Thermodynamics control a reaction when there are two products under different reaction conditions. The Kinetic product (Product A) will be formed fast, and the Thermodynamic product (Product B) will be formed more slowly. Usually the first product formed is the more stable favored product, but in this case, the slower product formed is the more stable product; Product B.
Introduction
Like nonconjugated dienes, conjugated dienes are subject to attack by electrophiles. In fact, conjugated electrophiles experience relatively greater kinetic reactivity when reacted with electrophiles than nonconjugated dienes do. Upon electrophilic addition, the conjugated diene forms a mixture of two products—the kinetic product and the thermodynamic product—whose ratio is determined by the conditions of reaction. A reaction yielding more thermodynamic product is under thermodynamic control, and likewise, a reaction that yields more kinetic product is under kinetic control.
Basic Reaction
Detailed Mechanism
Kinetic and Thermodynamic Products
Kinetic products form the fastest. They usually occur at or below 0°C. This is also known as the 1,2-adduct because the substituents are added to the first and second carbons. Kinetic products contain a terminal double bond and the reaction is irreversible. Thermodynamic products form at higher temperatures, generally greater than 40 °C. These are known are the 1,4-adducts because they add to the first and fourth carbons. Thermodynamic products contain an internal double bond and the reaction is reversible. Also, when reactions are carried out, thermodynamic products are more stable than kinetic products because they are more substituted.
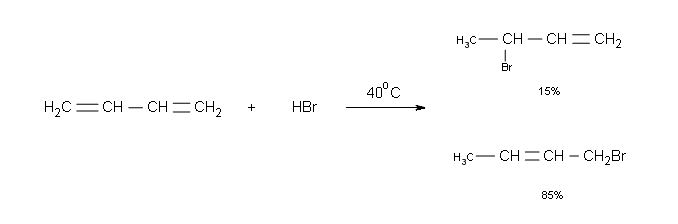
Figure 1: Conditions at 0 °C:
Final Products:
- 1,2 Addition (Kinetic controlled product)
- 1,4 Addition (Thermodynamic controlled product)
The reason for the two products is the difference in their activation energies. The reaction will go through to completion on the easiest path and in this case that is the Kinetic path. This path has a much lower Activation Energy which means that less is required to have the product formed. This product- Product A, is not the most stable form however. Over time, the amount of Product B- the more stable one, will increase and the Product A will decrease.
.gif?revision=1&size=bestfit&width=960&height=720)
Figure 2: Potential Energy profiles
Understanding the Reaction Coordinate Diagram
- Major product at low temperatures- 1, 2 Bromobutene: The main product at low temperatures is Kinetically controlled. This means that there is not enough energy to overcome the Activation Energy of the Thermodynamic product even though it is the more stable product.
- 98% product: At a low temperature the amount of energy in the reaction is not enough to get a large amount of the product over into the Thermodynamic Isomer. One of the two factors that influences the outcome of the reaction is the rate of product formation which is the Kinetic control. The Proximity Effect makes it so that the when the H is taken from the Br by the double bond, the Br is left close to the C2 and, in order to take away the positive charge, the C2 bonds with it quickly forming 1,2-Bromobutene.
- 2% product:The Thermodynamic Isomer is not favored at low temperatures because the Rate of Reaction is based on the Activation Energy. The Activation Energy of the Thermodynamic Isomer is higher than the Kinetic and therefore will happen a lot slower.
- The Intermediate is low in energy due to charge delocalization: The charge is delocalized across the three Carbons so the positive charge is not only on one Carbon. The Br will attack at the 2 and 4 positions.
- Kinetic Higher Energy product: The product formed Kinetically is less stable than the Thermodynamic product. The double bond in the Kinetic Product is only connected to one single bond which means that it has left of an Allylic effect on the overall structure.
- Thermodynamic Lower Energy Product: The Thermodynamic product has two Alkyl groups bonded to the double bond. This has a stabilizing effect on the molecule.
- Major Product at high temperatures- 1,4 bromobutene: This is the Thermodynamic Product that does not occur rapidly due to the Resonance Structure required to form the final product. If the reaction is left to go through equilibrium this product will predominate. It is not based on the physical conditions of the reaction and that is why it is the Thermodynamic product.
Table 1: Conjugated Dienese: Kinetics vs. Thermodynamic Conditions
.gif?revision=1&size=bestfit&width=768&height=384)
Kinetic and Thermodynamic Product Ratios
To ensure the greatest possible yield of thermodynamic products, the reaction should be carried out at a temperature of 40°C or greater. This is known as thermodynamic control. At higher temperatures and longer reaction times, thermodynamic products are favored. On the contrary, at lower temperatures, one would tend to see a greater yield of kinetic products. These products are generally formed at or around 0°C. Carrying out reactions around these temperatures is known as kinetic control and kinetic products form before thermodynamic products.
Since the thermodynamic product contains an internal double bond, it is more stable than the kinetic product, and this is due to hyperconjugation with neighboring atoms. Additionally, a higher activation energy results in the thermodynamic product forming slower than the kinetic product. Therefore, a thermodynamically controlled reaction gives a more stable product and kinetically controlled reaction gives a less stable product.
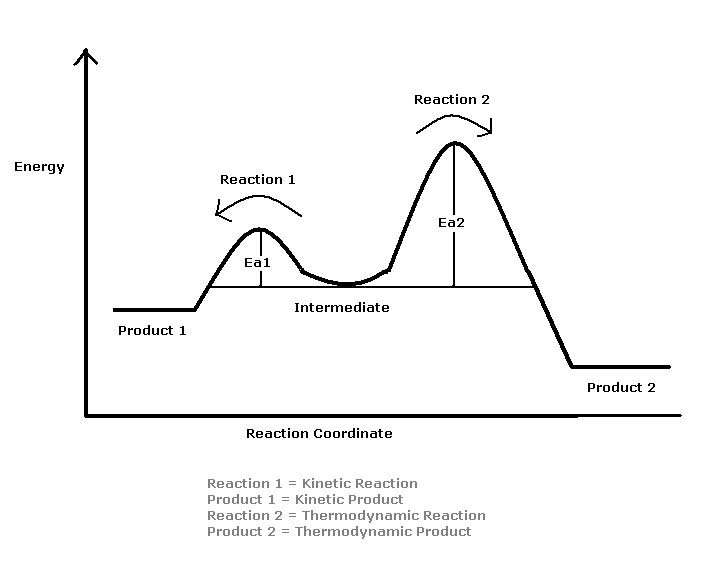
Figure: The various temperature conditions that may instigate a change or speed up the reaction
The conjugated diene has 2 double bonds with one single C-C bond between them. This structure offers stability because the two pi bonds can transfer electrons through the two carbons that are sp2 hybridized with a single bond which results in electron delocalization. Extended P orbital sharing makes this diene more stable than the isolated dienes. The more stable molecule also has lower energy and a shorter bond length.
Figure 4:
The p electrons reach out to the electrophile and form a bond that in turn forms a Carbocation. The Markovnikov Addition states that the most stable carbocation is most likely to be formed with the charge going on the more substituted carbon. The difference between a conjugated diene and an alkene is that there is still a double bond left after the reaction has completed.
- The Kinetic product is formed more quickly because it has a more stable carbocation to begin with. Due to resonance forms, the most stable isomer is the one with the double bond in the center of the molecule. As the reaction completes under normal conditions, the more likely product is the one formed with the most stable carbocation.
- The Kinetic isomer product. While the carbocation is more stable, the final product is not. The double bond ends up on a primary carbon. It would have more stabilizing effect if it were in the middle which is why it tries to get there.
Conclusion
The reactivity of conjugated dienes (hydrocarbons that contain two double bonds) varies depending on the location of double bonds and temperature of the reaction.These reactions can produce both thermodynamic and kinetic products. Isolated double bonds provide dienes with less stability thermodynamically than conjugated dienes. However, they are more reactive kinetically in the presence of electrophiles and other reagents. This is a result of Markovnikov addition to one of the double bonds. A carbocation is formed after a double bond is opened. This carbocation has two resonance structures and addition can occur at either of the positive carbons.
References
- Elsheimer, Seth R. Introduction to Organic Chemistry. Blackwell Publishing. 2000. p 136-139.
- Vollhardt,Peter, Schore, Neil. Organic Chemistry Structure and Function. W. H. Freeman and Co. New York. 2007. P 614- 618.
- Silberberg, Martin S., Chemistry; The Molecular Nature of Matter and Change. McGraw Hill. 2nd Ed. 2000.
Practice Problems
- Write out the products of 1,2 addition and 1,4- addition of a) HBr and Br. b) DBr to 1,3-cyclo-hexadiene. What is unusual about the products of 1,2- and 1,4- addition of HX to unsubstituted cyclic 1,3-dienes?
- Is the 1,2-addition product formed more rapidly at higher temperatures, even though it is the 1,4-addition product that predominates under these conditions?
- Why is the 1,4-addition product the thermodynamically more stable product?
- Out of the following radical cations which one is not a reasonable resonance structure?

5. Addition of 1 equivalent of Bromine to 2,4-hexadiene at 0 degrees C gives 4,5-dibromo-2-hexene plus an isomer. Which of the following is that isomer:
- 5,5-dibromo-2-hexene
- 2,5-dibromo-3-hexene
- 2,2-dibromo-3-hexene
- 2,3-dibromo-4-hexene
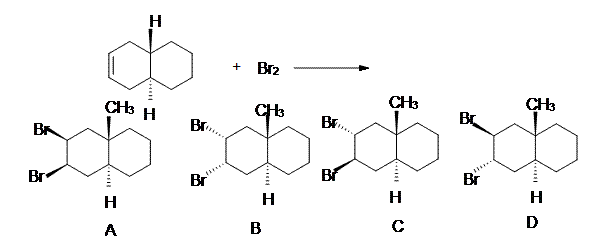
6. Which of the following will be the kinetically favored product from the depicted reaction?
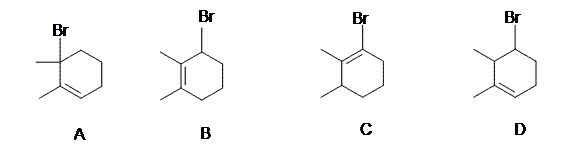
7. Addition of HBr to 2,3-dimethyl-1,3-cyclohexadiene may occur in the absence or presence of peroxides. In each case two isomeric C8H13Br products are obtained. Which of the following is a common product from both reactions?
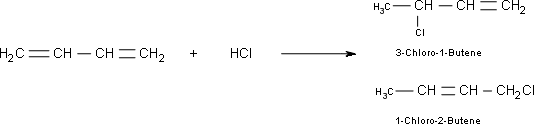
8. and 9.
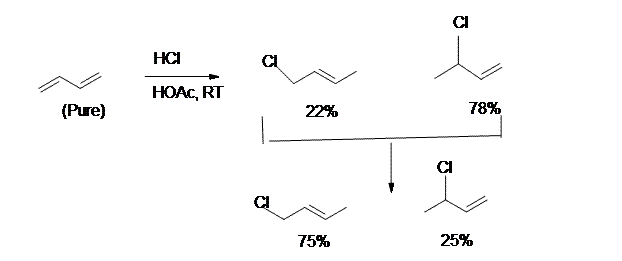
8. The kinetically controlled product in the above reaction is:
- 3-Chloro-1-Butene
- 1-Chloro-2-Butene
9. For the reaction in question 8, which one is the result of 1,4-addition?
- 3-Chloro-1-Butene
- 1-Chloro-2-Butene
Answers to Problems
1. A) Same product for both modes of addition.

B) Both cis and trans isomers will form.
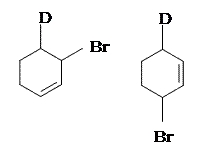
Addition of the HX to unsubstituted cycloalka-1,3-dienes in either 1,2- or 1,4- manner gives the same product becasuse of symmetry.
2. Yes. the Kinetic Product will still form faster but in this case there will be enough energy to form the thermodynamic product because the thermodynamic product is still more stable.
3. The 1,4- product is more thermodynamically stable because there are two alkyl groups on each side of the double bond. This form offers stability to the overall structure.
4. All of these isomers are viable.
5. D
6. C
7. D
8. A
9. B
Contributors
- Natasha Singh