Multiple Stereogenic Centers
- Page ID
- 1252
Two or More Chiral Centers
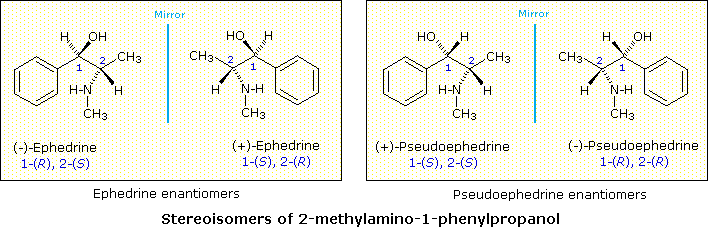
The Chinese shrub Ma Huang (Ephedra vulgaris) contains two physiologically active compounds ephedrine and pseudoephedrine. Both compounds are stereoisomers of 2-methylamino-1-phenyl-1-propanol, and both are optically active, one being levorotatory and the other dextrorotatory. Since the properties of these compounds (see below) are significantly different, they cannot be enantiomers. How, then, are we to classify these isomers and others like them?
| Ephedrine from Ma Huang: | m.p. 35 - 40 º C, [α]D = –41º, moderate water solubility | This isomer may be referred to as (–)-ephedrine |
| Pseudoephedrine from Ma Huang: | m.p. 119 º C, [α]D = +52º, relatively insoluble in water | This isomer may be referred to as (+)-pseudoephedrine |
Since these two compounds are optically active, each must have an enantiomer. Although these missing stereoisomers were not present in the natural source, they have been prepared synthetically and have the expected identical physical properties and opposite-sign specific rotations with those listed above. The structural formula of 2-methylamino-1-phenylpropanol has two stereogenic carbons (#1 & #2). Each may assume an R or S configuration, so there are four stereoisomeric combinations possible. These are shown in the following illustration, together with the assignments that have been made on the basis of chemical interconversions.
As a general rule, a structure having n chiral centers will have 2n possible combinations of these centers. Depending on the overall symmetry of the molecular structure, some of these combinations may be identical, but in the absence of such identity, we would expect to find 2n stereoisomers. Some of these stereoisomers will have enantiomeric relationships, but enantiomers come in pairs, and non-enantiomeric stereoisomers will therefore be common. We refer to such stereoisomers as diastereomers. In the example above, either of the ephedrine enantiomers has a diastereomeric relationship with either of the pseudoephedrine enantiomers.
For an interesting example illustrating the distinction between a chiral center and an asymmetric carbon Click Here.
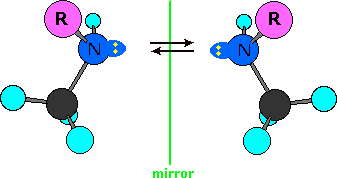
Stereogenic Nitrogen
A close examination of the ephedrine and pseudoephedrine isomers suggests that another stereogenic center, the nitrogen, is present. As noted earlier, single-bonded nitrogen is pyramidal in shape, with the non-bonding electron pair pointing to the unoccupied corner of a tetrahedral region. Since the nitrogen in these compounds is bonded to three different groups, its configuration is chiral. The non-identical mirror-image configurations are illustrated in the following diagram (the remainder of the molecule is represented by R, and the electron pair is colored yellow). If these configurations were stable, there would be four additional stereoisomers of ephedrine and pseudoephedrine. However, pyramidal nitrogen is normally not configurationally stable. It rapidly inverts its configuration (equilibrium arrows) by passing through a planar, sp2-hybridized transition state, leading to a mixture of interconverting R and S configurations. If the nitrogen atom were the only chiral center in the molecule, a 50:50 (racemic) mixture of R and S configurations would exist at equilibrium. If other chiral centers are present, as in the ephedrin isomers, a mixture of diastereomers will result. In any event, nitrogen groups such as this, if present in a compound, do not contribute to isolable stereoisomers.
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry