Reactions of Carboxylic Acids
- Page ID
- 783
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Salt Formation
Because of their enhanced acidity, carboxylic acids react with bases to form ionic salts, as shown in the following equations. In the case of alkali metal hydroxides and simple amines (or ammonia) the resulting salts have pronounced ionic character and are usually soluble in water. Heavy metals such as silver, mercury and lead form salts having more covalent character (3rd example), and the water solubility is reduced, especially for acids composed of four or more carbon atoms.
| RCO2H | + | NaHCO3 |  |
RCO2(–) Na(+) + CO2 + H2O |
| RCO2H | + | (CH3)3N: |  |
RCO2(–) (CH3)3NH(+) |
| RCO2H | + | AgOH |  |
RCO2δ(-) Agδ(+) + H2O |
Carboxylic acids and salts having alkyl chains longer than six carbons exhibit unusual behavior in water due to the presence of both hydrophilic (CO2) and hydrophobic (alkyl) regions in the same molecule. Such molecules are termed amphiphilic (Gk. amphi = both) or amphipathic. Depending on the nature of the hydrophilic portion these compounds may form monolayers on the water surface or sphere-like clusters, called micelles, in solution.
Substitution of the Hydroxyl Hydrogen
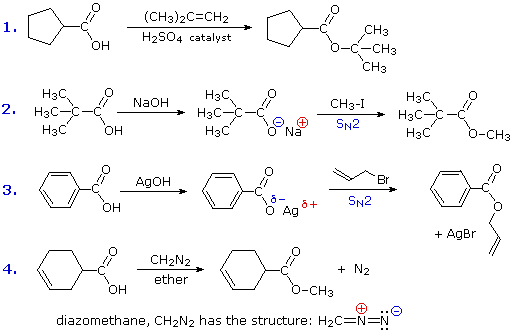
This reaction class could be termed electrophilic substitution at oxygen, and is defined as follows (E is an electrophile). Some examples of this substitution are provided in equations (1) through (4).
| RCO2–H + E(+) |  |
RCO2–E + H(+) |
If E is a strong electrophile, as in the first equation, it will attack the nucleophilic oxygen of the carboxylic acid directly, giving a positively charged intermediate which then loses a proton. If E is a weak electrophile, such as an alkyl halide, it is necessary to convert the carboxylic acid to the more nucleophilic carboxylate anion to facilitate the substitution. This is the procedure used in reactions 2 and 3. Equation 4 illustrates the use of the reagent diazomethane (CH2N2) for the preparation of methyl esters. This toxic and explosive gas is always used as an ether solution (bright yellow in color). The reaction is easily followed by the evolution of nitrogen gas and the disappearance of the reagent's color. This reaction is believed to proceed by the rapid bonding of a strong electrophile to a carboxylate anion.
The nature of SN2 reactions, as in equations 2 & 3, has been described elsewhere. The mechanisms of reactions 1 & 4 will be displayed by clicking the "Toggle Mechanism" button below the diagram.
Alkynes may also serve as electrophiles in substitution reactions of this kind, as illustrated by the synthesis of vinyl acetate from acetylene. Intramolecular carboxyl group additions to alkenes generate cyclic esters known as lactones. Five-membered (gamma) and six-membered (delta) lactones are most commonly formed. Electrophilic species such as acids or halogens are necessary initiators of lactonizations. Even the weak electrophile iodine initiates iodolactonization of γ,δ- and δ,ε-unsaturated acids. Examples of these reactions will be displayed by clicking the "Other Examples" button.
Substitution of the Hydroxyl Group
Reactions in which the hydroxyl group of a carboxylic acid is replaced by another nucleophilic group are important for preparing functional derivatives of carboxylic acids. The alcohols provide a useful reference chemistry against which this class of transformations may be evaluated. In general, the hydroxyl group proved to be a poor leaving group, and virtually all alcohol reactions in which it was lost involved a prior conversion of –OH to a better leaving group. This has proven to be true for the carboxylic acids as well.
Four examples of these hydroxyl substitution reactions are presented by the following equations. In each example, the new bond to the carbonyl group is colored magenta and the nucleophilic atom that has replaced the hydroxyl oxygen is colored green. The hydroxyl moiety is often lost as water, but in reaction #1 the hydrogen is lost as HCl and the oxygen as SO2. This reaction parallels a similar transformation of alcohols to alkyl chlorides, although its mechanism is different. Other reagents that produce a similar conversion to acyl halides are PCl5 and SOBr2.
The amide and anhydride formations shown in equations #2 & 3 require strong heating, and milder procedures that accomplish these transformations will be described in the next chapter.
A thoughtful examination of this reaction (#4) leads one to question why it is classified as a hydroxyl substitution rather than a hydrogen substitution. The following equations, in which the hydroxyl oxygen atom of the carboxylic acid is colored red and that of the alcohol is colored blue, illustrate this distinction (note that the starting compounds are in the center).
H2O + CH3CO-OCH2CH3 |
H-substitution |
|
HO-substitution |
CH3CO-OCH2CH3 + H2O |
In order to classify this reaction correctly and establish a plausible mechanism, the oxygen atom of the alcohol was isotopically labeled as 18O (colored blue in our equation). Since this oxygen is found in the ester product and not the water, the hydroxyl group of the acid must have been replaced in the substitution. A mechanism for this general esterification reaction will be displayed on clicking the "Esterification Mechanism" button; also, once the mechanism diagram is displayed, a reaction coordinate for it can be seen by clicking the head of the green "energy diagram" arrow. Addition-elimination mechanisms of this kind proceed by way of tetrahedral intermediates (such as A and B in the mechanism diagram) and are common in acyl substitution reactions. Acid catalysis is necessary to increase the electrophilic character of the carboxyl carbon atom, so it will bond more rapidly to the nucleophilic oxygen of the alcohol. Base catalysis is not useful because base converts the acid to its carboxylate anion conjugate base, a species in which the electrophilic character of the carbon is reduced.
Since a tetrahedral intermediate occupies more space than a planar carbonyl group, we would expect the rate of this reaction to be retarded when bulky reactants are used. To test this prediction the esterification of acetic acid was compared with that of 2,2-dimethylpropanoic acid, (CH3)3CO2H. Here the relatively small methyl group of acetic acid is replaced by a larger tert-butyl group, and the bulkier acid reacted fifty times slower than acetic acid. Increasing the bulk of the alcohol reactant results in a similar rate reduction.
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry


