Oxidation States of Nitrogen
- Page ID
- 765
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In comparing the chemistry of the amines with alcohols and ethers, we discover many classes of related compounds in which nitrogen assumes higher oxidation states, in contrast to limited oxidation states of oxygen.
In this context, keep in mind that the oxidation state of elemental oxygen (O2) and nitrogen (N2) is defined as zero. The most prevalent state of covalently bonded oxygen is -2. This is the case for water, alcohols, ethers and carbonyl compounds. The only common higher oxidation state (-1) is found in the peroxides, R–O–O–R, where R=hydrogen, alkyl, aryl or acyl. Because of the low covalent bond energy of the peroxide bond (ca.35 kcal/mole), these compounds are widely used as free radical initiators, and are sometimes dangerously explosive in their reactivity (e.g. triacetone triperoxide used by terrorist bombers). Nitrogen compounds, on the other hand, encompass oxidation states of nitrogen ranging from -3, as in ammonia and amines, to +5, as in nitric acid. The following table lists some of the known organic compounds of nitrogen, having different oxidation states of that element. Some of these classes of compounds have been described; others will be discussed later.
|
Oxidation State |
_3 |
_2 |
_1 |
0 |
+1 |
+3 |
|---|---|---|---|---|---|---|
| Formulas (names) |
R3N (amines) (ammonium) (imines) (nitriles) |
R2N–NR2 (hydrazines) (hydrazones) |
RN=NR (azo cpd.) (hydroxyl amine) (amine oxide) |
N2 (nitrogen) (diazonium) |
R–N=O (nitroso) |
R-NO2 (nitro) (nitrite ester) |
Amine Oxides and the Cope Elimination
Amine oxides are prepared by oxidizing 3º-amines or pyridines with hydrogen peroxide or peracids (e.g. ZOOH, where Z=H or acyl).
\[R_3N: + ZOOH \rightarrow R_3N^{(+)}–O^{(–)} + ZOH\]
Amine oxides are relatively weak bases, pKa ca. 4.5, compared with the parent amine. The coordinate covalent N–O function is polar, with the oxygen being a powerful hydrogen bond acceptor. If one of the alkyl substituents consists of a long chain, such as C12H25, the resulting amine oxide is an amphoteric surfactant and finds use in shampoos and other mild cleaning agents.
An elimination reaction, complementary to the Hofmann elimination, occurs when 3º-amine oxides are heated at temperatures of 150 to 200 ºC. This reaction is known as the Cope Elimination. It is commonly carried out by dropwise addition of an amine oxide solution to a heated tube packed with small glass beads. A stream of nitrogen gas flowing through the column carries the volatile alkene products to a chilled receiver. The nitrogen-containing product is a hydroxyl amine. Unlike the Hofmann elimination, this reaction takes place by a concerted cyclic reorganization, as shown in the following diagram. For such a mechanism, the beta-hydrogen and amine oxide moieties necessarily have a syn-relationship.
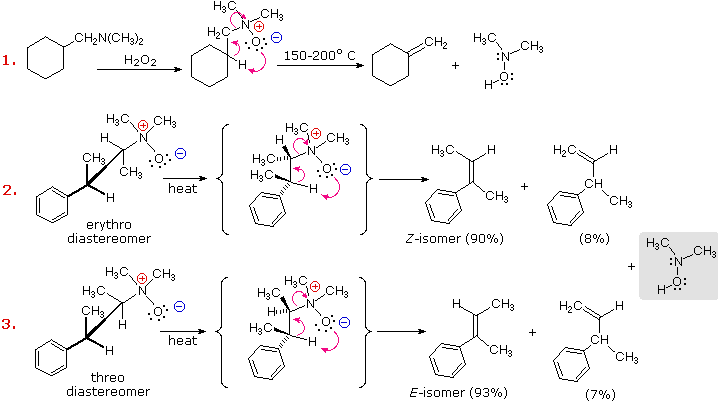
Cope elimination of diastereomeric amine oxides, such as those shown in examples #2 & 3 above, provide proof of the syn-relationship of the beta-hydrogen and amine oxide groups. These examples also demonstrate a strong regioselectivity favoring the more stable double bond.
Pyrolytic syn-Eliminations
Amine oxides are not the only functions that undergo a unimolecular syn-elimination on heating. To see examples of other cases Click Here
Nitroxide Radicals
2º-Amines lacking α-hydrogens are oxidized by peroxides (ZOOH) to nitroxide radicals of surprising stability. In the example shown at the top of the following diagram it should be noted that resonance delocalization of the unpaired electron contributes to a polar N–O bond. The R=H compound, known by the acronym TEMPO, is a relatively stable red solid. Many other nitroxides have been prepared, three of which are drawn at the lower right. If one or more hydrogens are present on an adjacent carbon, the nitroxide decomposes to mixtures including amine oxides and nitrones, as shown at the lower left. Nitroxides are oxidized to unstable oxammonium cations by halogens.
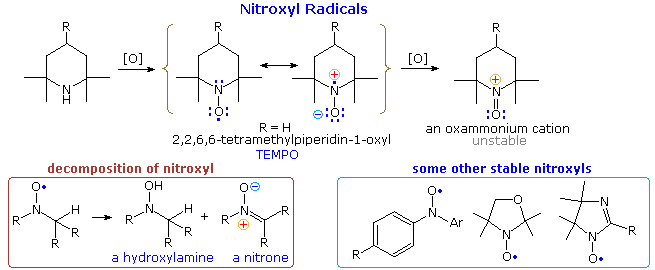
The spin of the nitroxyl unpaired electron may be studied by a technique called electron paramagnetic resonance (epr or esr). Experiments of this kind have demonstrated that the epr spectra are sensitive to substituents on the radical as well as its immediate environment. This has led to a spin labeling strategy for investigating the conformational structures of macromolecules like proteins. Thus, site-directed spin labeling (SDSL) has emerged as a valuable technique for mapping elements of secondary structure, at the level of the backbone fold, in a wide range of proteins, including those not amenable to structural characterization using classical structural techniques, such as nuclear magnetic resonance and X-ray crystallography.
Phosphorous Analogs of Amines
Phosphorus is beneath nitrogen in the periodic table. To see examples of organophosphorus compounds and their chemistry Click Here
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry


