Nuclear Magnetic Resonance (NMR) of Alkenes
- Page ID
- 873
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In the previous section, we learned about the physical properties of alkenes (Physical Properties of Alkenes). The double bonded carbons in alkene molecules also have an effect of shifts shown in 1H and 13C nuclear magnetic resonance spectr.
Hydrogens near double bonds are deshielded
For background information of 1H NMR, you can refer 1H Nuclear Magnetic Resonance from the last chapter. In 1H NMR spectrum, hydrogen atoms bound to a carbon consisting of a double bond (these hydrogens are called alkenyl hydrogens) are typically found in low field of the NMR spectrum, which is the left side, and the hydrogens are said to be deshielded. The cause for this is due to the movement of the electrons in the pi bond of the carbon-carbon double bond.
Alkenyl hydrogens create an external magnetic field that is perpendicular to the double bond axis and causes the electrons in the pi bond to enter a circular motion (shown in red). The circular motion actually reinforces the external field at the edge of the double bond on both sides of the pi bond but creates a local field (shown in purple and green) that opposes the external field in the center of the double bond. Because of this pulling force within the pi bond across the double bond which reinforces the regions occupied by alkenyl hydrogens, the alkenyl hydrogens are strongly deshielded.
Additionally, alkenyl hydrogens do not have to be all chemical-shift equivalent, and when they aren't, coupling will be observed which is the different peaks in an MNR spectrum.
Cis and trans coupling appear differently on 1H NMR spectrum
Here are a couple of terms to know:
- Vicinal - Coupling between hydrogens on adjacent carbons.
- Geminal - Coupling between nonequivalent hydrogens on the same carbon atom.
| Type of coupling | Name | Range (Hz) | Typical (Hz) |
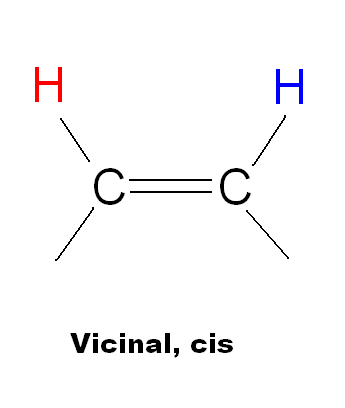 |
Vicinal, cis | 6-14 | 10 |
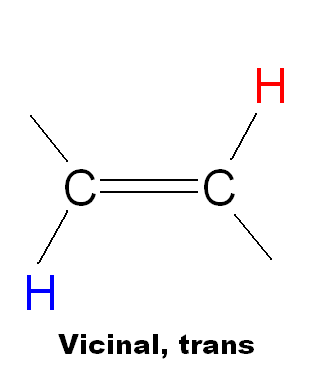 |
Vicinal, trans | 11-18 | 16 |
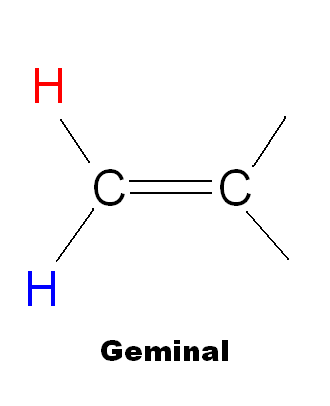 |
Geminal | 0-3 | 2 |
|
|
None | 4-10 | 6 |
 |
Allylic | 0.5-3.0 | 2 |
When alkynel hydrogen atoms are not symmetrically substituted on a double bonded carbon, the hydrogens of a cis and trans isomer will yield a different shift on the NMR spectrum. Because the coupling constant is smaller in a cis isomer than in a trans isomer, the NMR spectrums of the two isomers are different conveying the hydrogens in a cis isomer to be slightly more upfield to-- the right of the spectrum-- and trans hydrogens to be more downfield to the left.

Sometimes coupling will lead to very complicated patterns as a result of the J values that vary widely due to the relationship between the hydrogens involved. When this occurs, information can still be derived to determine the structure of a molecule by looking at the number of signals, the chemical shift of each one, integration, and splitting patterns similarly to identifying alkane NMR.
Alkenyl carbons are deshielded in 13C NMR
For background information on 13C NMR, please refer to 13C Nuclear Magnetic Resonance from the previous chapter. Compared to alkane carbons with one bond, alkene carbons show a relatively low field shift on the 13C NMR spectrum and absorb about 100 ppm lower field. Also, in broad-band decoupled 13C NMR, sp2 carbons absorb as sharp single lines so with these two methods, it is easy to determine the presence of a double bond in 13C NMR spectrum.
Here are the common 13C Chemical Shift Ranges:
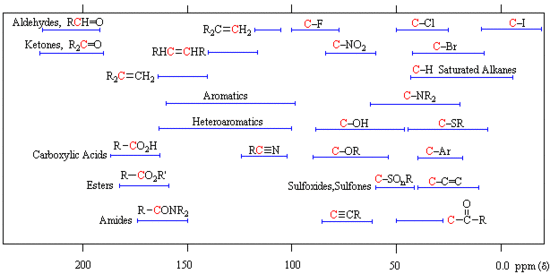
Note that the carbon-carbon double bonds are found in the range between 100-170 ppm. Carbon atoms on alkenes that are attatched to another carbon group are found more downfield than carbon alkenes attatched to hydrogens.
Let's try a 1H NMR practice problem with C4H7Cl:

Remember from previous sections that to solve an NMR spectrum with double bonds, we must know the Degrees of Unsaturation. From this, we get degrees of unsaturation= (9-7)/2=1 so there is one pi bond or ring in our molecule. Next we must look at the integration of the NMR spectrums.
- ppm= 1.8 with 3H reveals a CH3attached to an unsaturated functional group
- ppm= 4.0 with integration of 2H is a CH2 most likely attached to the Cl
- ppm = 4.9 and 5.1 are singlets of 1H and must be our two alkene hydrogens
There are three ways to attach the discovery we made, but only one of them are the correct answer. Since the coupling of the two alkene hydrogens are small whereas vicinal hydrogens tend to be large, we conclude that the hydrogens are geminal and appear on the same carbon. This leaves the two other groups to be located on the other alkene carbon.
References
- Vollhardt, Peter K and Neil E. Schore. Organic Chemistry Structure and Function. Ed. Clancy Marshall. New York: W.H. Freeman and Company, 2007.
- Wade, L.G. (Sixth Ed., 2006). Organic Chemistry. Pearson Prentice Hall.
- Saunders, W. H. (1964). Patai, Saul. ed. The Chemistry of Alkenes. Wiley Interscience.
Practice Problems
- If an alkene isomer showed coupling at 17 J (Hz), would it be cis or trans?
- Why are alkenyl hydrogens deshielded?
- Rank the order of coupling from highest to lowest range:
- vicinal, cis
- vicinal, trans
- geminal
- True or false: In 13C NMR, alkene carbons show an upfield shift compared to alkane carbons.
- What are two ways to distinguish an alkene in a 13C NMR spectrum?
Solutions
- trans because the range of trans coupling in an alkene is 11-18 J (Hz) while cis is 6-14 J (Hz)
- Alkenyl hydrogens are deshielded due to the movement of the electrons in the pi bond. Alkenyl hydrogens create an external magnetic field that is perpendicular to the double bond axis and causes the electrons in the pi bond to enter a circular motion. The circular motion of the pi bonds reinfornce the external field where the hydrogens are located thus being strongly deshielded and appearing low field in an NMR spectrum.
- b) vicinal, trans > a) vicinal, cis > c) geminal
- False. Alkene carbons absorb at about 100 ppm lower field than alkane carbons thus are found low field in a 13C NMR spectrum.
- Alkenes typically absorb around 122 ppm and appear as sharp lines in 13C NMR spectrums making them easy to distinguish.