Reactions of Acyl Chlorides Involving Nitrogen Compounds
- Page ID
- 3934
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This page discusses the reactions of acyl chlorides (acid chlorides) with ammonia and primary amines. These reactions are considered together because their chemistry is so similar.
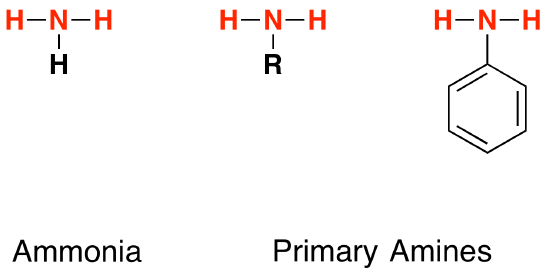
Comparing the structures of ammonia and primary amines
Each of these substances contain an -NH2 group. In ammonia, this is attached to a hydrogen atom. In a primary amine, it is attached to an alkyl group (shown by "R" in the diagram below) or a benzene ring.
Reactions with acyl chlorides
Taking a general case of a reaction between ethanoyl chloride and a compound XNH2 (where X is hydrogen, an alkyl group, or a benzene ring). The reaction happens in two stages:
First:
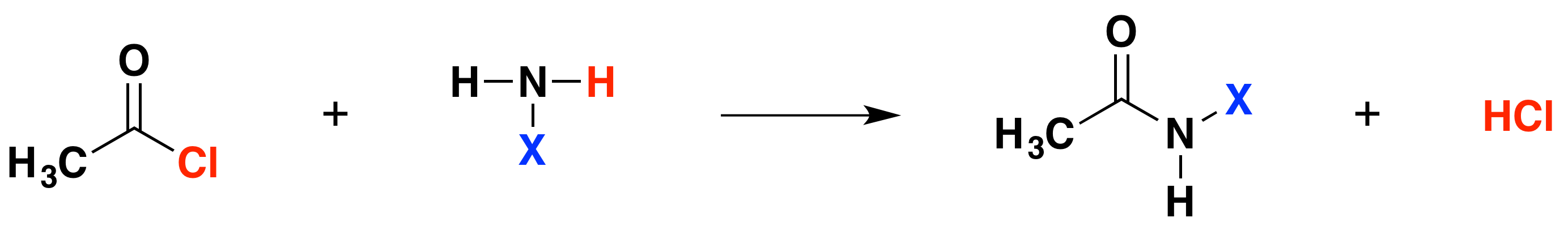

In each case, hydrogen chloride gas is initially formed, the hydrogen coming from the -NH2 group, and the chlorine from the ethanoyl chloride. The remaining species join together. However, ammonia and amines are basic, and react with hydrogen chloride to produce a salt. Therefore, the second stage of the reaction is the following:
\[ XNH_2 + HCl \rightarrow XNH_3^+ Cl^-\]
This is illustrated with specific compounds below.
The individual reactions
The reaction with ammonia
In this case, the "X" in the equations above is a hydrogen atom. In the first instance, hydrogen chloride gas and an organic compound called an amide are formed. An amide contains the group -CONH2. In the reaction between ethanoyl chloride and ammonia, the amide formed is called ethanamide:
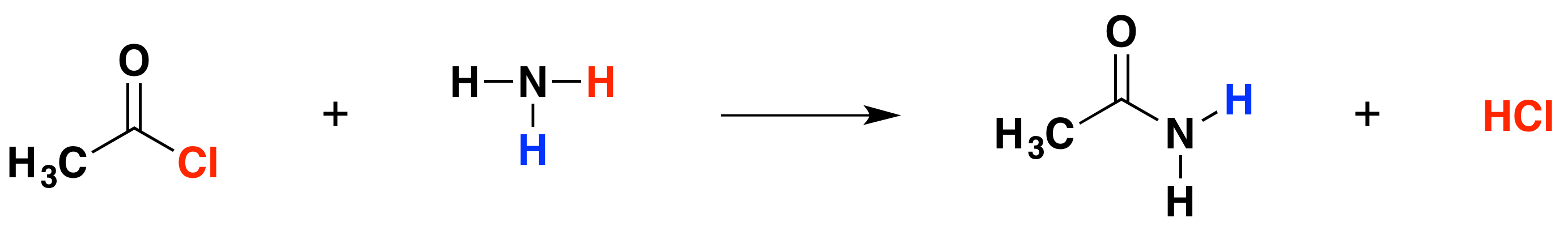
This reaction is more commonly written as follows:

The hydrogen chloride produced reacts with excess ammonia to form ammonium chloride:

These equations can be combined to give the overall equation:

The ethanoyl chloride is typically added to a concentrated solution of ammonia in water. This is a violent reaction producing large amounts of white smoke, a mixture of solid ammonium chloride and ethanamide. Some of the mixture remains dissolved in water as a colorless solution.
The reaction with primary amines
The reaction with methylamine
Consider methylamine as a typical primary amine, in which the -NH2 is attached to an alkyl group. The initial equation would be the following:
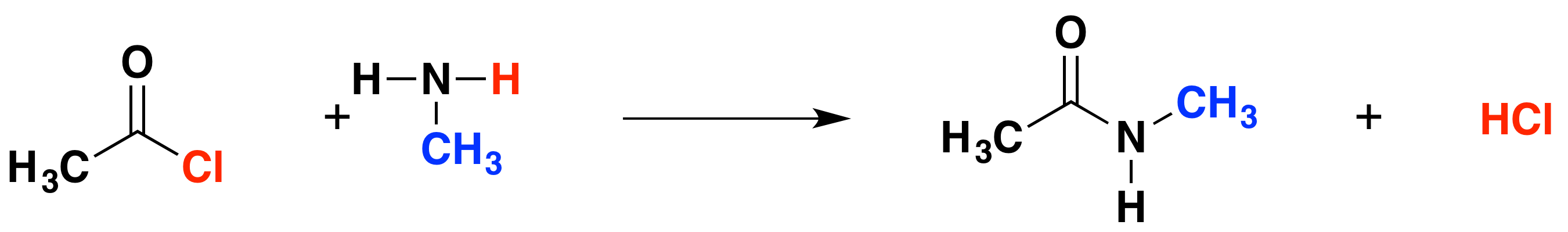
The organic product in this case is called an N-substituted amide. Compare the structure with the amide produced in the reaction with ammonia, the only difference is that one of the hydrogen atoms on the nitrogen has been substituted for a methyl group. This particular compound is N-methylethanamide. The "N" indicates that the substitution is on the nitrogen atom, and not elsewhere in the molecule.
This reaction equation is typically be written the following way:
\[ CH_3COCl + CH_3NH_2 \rightarrow CH_3CONHCH_3 + HCl\]
Primary amines can be thought of as modified ammonia molecules. If ammonia is basic and forms a salt with the hydrogen chloride, excess methylamine does exactly the same thing:
\[ CH_3NH_2 + HCl \rightarrow CH_3NH_3^+ \; Cl^-\]
The salt is called methylammonium chloride. It is like ammonium chloride, except that one of the hydrogens has been replaced by a methyl group. These two equations are combined into one overall equation for the reaction, as shown below:
\[ CH_3COCl + 2CH_3NH_2 \rightarrow CH_3CONHCH_3 + CH_3NH_3Cl\]
The reaction looks identical to the ammonia reaction. The methylamine is again used as a concentrated aqueous solution. There is a violent reaction producing a white solid mixture of N-methylethanamide and methylammonium chloride.
The reaction with phenylamine (aniline)
Phenylamine is the simplest primary amine in which the -NH2 group is attached directly to a benzene ring. Its old name is aniline. In phenylamine, no other groups are attached to the ring. The formula of phenylamine is C6H5NH2. There is no essential difference between this reaction and the methylamine reaction, except that phenylamine is a brown liquid, and the solid products tend to be stained brown.
The overall equation for the reaction is given below:
\[ CH_3COCl + 2C_6H_5NH_2 \rightarrow CH_3CONHC_6H_5 + C_6H_5NH_3Cl\]
The products are N-phenylethanamide and phenylammonium chloride. This reaction is often confusing if the phenylamine is drawn showing the benzene ring, and especially if the reaction is examined from the point of view of the phenylamine. For example, the product molecule might be drawn looking like this:
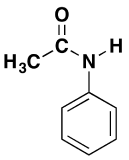
This is obviously the same molecule as in the equation above, but it emphasizes the phenylamine part. Notice that one of the hydrogen atoms of the -NH2 group has been replaced by an acyl group, an alkyl group attached to a carbon-oxygen double bond. Phenylamine can be said to have been acylated or undergone acylation. Because of the nature of this particular acyl group, it is also described as ethanoylation. The hydrogen atom is replaced by an ethanoyl group, CH3CO-.
Contributors
Jim Clark (Chemguide.co.uk)


