Ionic and Covalent Bonds
- Page ID
- 839
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)There are many types of chemical bonds and forces that bind molecules together. The two most basic types of bonds are characterized as either ionic or covalent. In ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron donor and one electron acceptor. In contrast, atoms with the same electronegativity share electrons in covalent bonds, because neither atom preferentially attracts or repels the shared electrons.
Introduction
Ionic bonding is the complete transfer of valence electron(s) between atoms. It is a type of chemical bond that generates two oppositely charged ions. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. Ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal.
Ionic bonding is observed because metals have few electrons in their outer-most orbitals. By losing those electrons, these metals can achieve noble gas configuration and satisfy the octet rule. Similarly, nonmetals that have close to 8 electrons in their valence shells tend to readily accept electrons to achieve noble gas configuration. In ionic bonding, more than 1 electron can be donated or received to satisfy the octet rule. The charges on the anion and cation correspond to the number of electrons donated or received. In ionic bonds, the net charge of the compound must be zero.
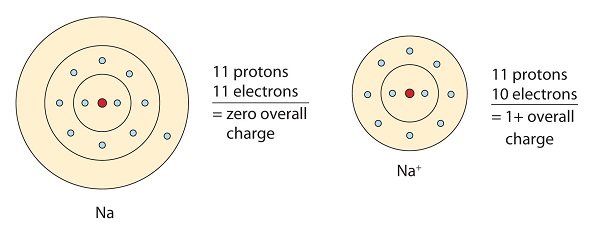
This sodium molecule donates the lone electron in its valence orbital in order to achieve octet configuration. This creates a positively charged cation due to the loss of electron.
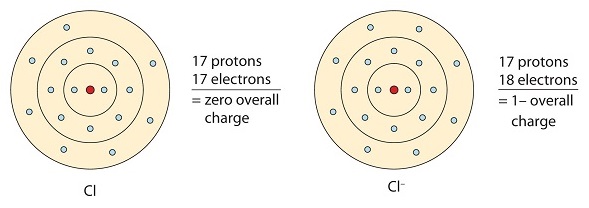
This chlorine atom receives one electron to achieve its octet configuration, which creates a negatively charged anion.
The predicted overall energy of the ionic bonding process, which includes the ionization energy of the metal and electron affinity of the nonmetal, is usually positive, indicating that the reaction is endothermic and unfavorable. However, this reaction is highly favorable because of the electrostatic attraction between the particles. At the ideal interatomic distance, attraction between these particles releases enough energy to facilitate the reaction. Most ionic compounds tend to dissociate in polar solvents because they are often polar. This phenomenon is due to the opposite charges on each ion.
.jpg?revision=2)
In this example, the sodium atom is donating its 1 valence electron to the chlorine atom. This creates a sodium cation and a chlorine anion. Notice that the net charge of the resulting compound is 0.
In this example, the magnesium atom is donating both of its valence electrons to chlorine atoms. Each chlorine atom can only accept 1 electron before it can achieve its noble gas configuration; therefore, 2 atoms of chlorine are required to accept the 2 electrons donated by the magnesium. Notice that the net charge of the compound is 0.
Covalent Bonding
Covalent bonding is the sharing of electrons between atoms. This type of bonding occurs between two atoms of the same element or of elements close to each other in the periodic table. This bonding occurs primarily between nonmetals; however, it can also be observed between nonmetals and metals.
If atoms have similar electronegativities (the same affinity for electrons), covalent bonds are most likely to occur. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to achieve octet configuration and become more stable. In addition, the ionization energy of the atom is too large and the electron affinity of the atom is too small for ionic bonding to occur. For example: carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. To form ionic bonds, Carbon molecules must either gain or lose 4 electrons. This is highly unfavorable; therefore, carbon molecules share their 4 valence electrons through single, double, and triple bonds so that each atom can achieve noble gas configurations. Covalent bonds include interactions of the sigma and pi orbitals; therefore, covalent bonds lead to formation of single, double, triple, and quadruple bonds.
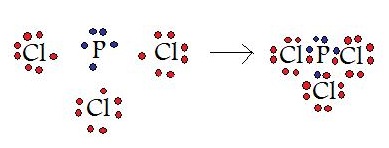
In this example, a phosphorous atom is sharing its three unpaired electrons with three chlorine atoms. In the end product, all four of these molecules have 8 valence electrons and satisfy the octet rule.
Bonding in Organic Chemistry
Ionic and covalent bonds are the two extremes of bonding. Polar covalent is the intermediate type of bonding between the two extremes. Some ionic bonds contain covalent characteristics and some covalent bonds are partially ionic. For example, most carbon-based compounds are covalently bonded but can also be partially ionic. Polarity is a measure of the separation of charge in a compound. A compound's polarity is dependent on the symmetry of the compound and on differences in electronegativity between atoms. Polarity occurs when the electron pushing elements, found on the left side of the periodic table, exchanges electrons with the electron pulling elements, on the right side of the table. This creates a spectrum of polarity, with ionic (polar) at one extreme, covalent (nonpolar) at another, and polar covalent in the middle.
Both of these bonds are important in organic chemistry. Ionic bonds are important because they allow the synthesis of specific organic compounds. Scientists can manipulate ionic properties and these interactions in order to form desired products. Covalent bonds are especially important since most carbon molecules interact primarily through covalent bonding. Covalent bonding allows molecules to share electrons with other molecules, creating long chains of compounds and allowing more complexity in life.
References
- Vollhardt, K. Peter C., and Neil E. Schore. Organic Chemistry Structure and Function. New York: W. H. Freeman, 2007.
- Petrucci, Ralph H. General Chemistry: Principles and Modern Applications. Upper Saddle River, NJ: Pearson Education, 2007.
- Brown, Theodore L., Eugene H. Lemay, and Bruce E. Bursten. Chemistry: The Central Science. 6th ed. Englewood Cliffs, NJ: Prentice Hall, 1994.
Problems
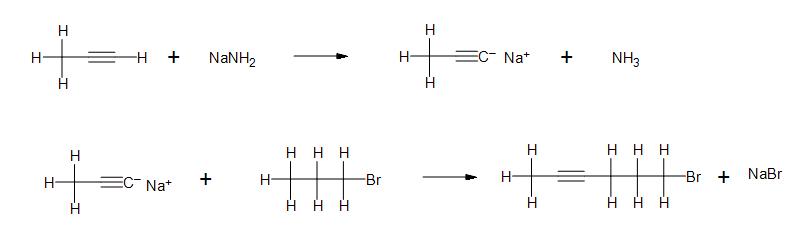
1. Are these compounds ionic or covalent?
2. In the following reactions, indicate whether the reactants and products are ionic or covalently bonded.
a)
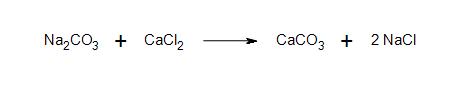
b) Clarification: What is the nature of the bond between sodium and amide? What kind of bond forms between the anion carbon chain and sodium?
c)
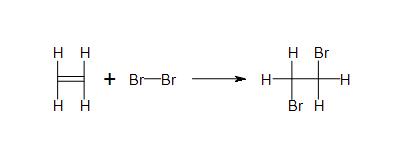
Solutions
- 1) From left to right: Covalent, Ionic, Ionic, Covalent, Covalent, Covalent, Ionic.
- 2a) All products and reactants are ionic.
- 2b) From left to right: Covalent, Ionic, Ionic, Covalent, Ionic, Covalent, Covalent, Ionic.
- 2c) All products and reactants are covalent.


.jpg?revision=2)