Alkene Stereoisomers
- Page ID
- 1248
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Configurational Stereoisomers of Alkenes
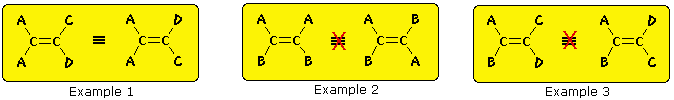
The carbon-carbon double bond is formed between two sp2 hybridized carbons, and consists of two occupied molecular orbitals, a sigma orbital and a pi orbital. Rotation of the end groups of a double bond relative to each other destroys the p-orbital overlap that creates the pi orbital or bond. Because the pi bond has a bond energy of roughly 60 kcal/mole, this resistance to rotation stabilizes the planar configuration of this functional group. As a result, certain disubstituted alkenes may exist as a pair of configurational stereoisomers, often designated cis and trans. The essential requirement for this stereoisomerism is that each carbon of the double bond must have two different substituent groups (one may be hydrogen). This is illustrated by the following general formulas. In the first example, the left-hand double bond carbon has two identical substituents (A) so stereoisomerism about the double bond is not possible (reversing substituents on the right-hand carbon gives the same configuration). In the next two examples, each double bond carbon atom has two different substituent groups and stereoisomerism exists, regardless of whether the two substituents on one carbon are the same as those on the other.
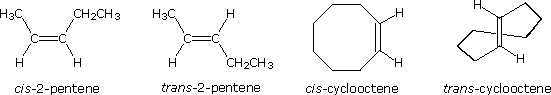
Some examples of this configurational stereoisomerism (sometimes called geometric isomerism) are shown below. Note that cycloalkenes smaller than eight carbons cannot exist in a stable trans configuration due to ring strain. A similar restriction holds against cycloalkynes smaller than ten carbons. Since alkynes are linear, there is no stereoisomerism associated with the carbon-carbon triple bond.
Nomenclature of Alkene Stereoisomers
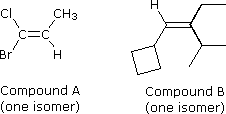
Configurational stereoisomers of the kind shown above need an additional nomenclature prefix added to the IUPAC name, in order to specify the spatial orientations of the groups attached to the double bond. Thus far, the prefixes cis- and trans- have served to distinguish stereoisomers; however, it is not always clear which isomer should be called cis and which trans. For example, consider the two compounds on the right. Both compound A (1-bromo-1-chloropropene) and compound B ( 1-cyclobutyl-2-ethyl-3-methyl-1-butene) can exist as a pair of configurational stereoisomers (one is shown). How are we to name these stereoisomers so that the configuration of each is unambiguously specified? Assignment of a cis or trans prefix to any of these isomers can only be done in an arbitrary manner, so a more rigorous method is needed. A completely unambiguous system, based on a set of group priority rules, assigns a Z (German, zusammen for together) or E (German, entgegen for opposite) to designate the stereoisomers. In the isomers illustrated above, for which cis-trans notation was adequate, Z is equivalent to cis and E is equivalent to trans.
The Sequence Rule for Assignment of Alkene Configurations
Assign priorities to double bond substituents by looking at the atoms attached directly to the double bond carbons.
1. The higher the atomic number of the immediate substituent atom, the higher the priority.
For example, H– < C– < N– < O– < Cl–. (priority increases left to right)
(Different isotopes of the same element are assigned a priority according to their atomic mass.)
2. If two substituents have the same immediate substituent atom, move to the next atom (away from the double bond) until a difference is found.
For example, CH3– < C2H5– < ClCH2– < BrCH2– < CH3O–.
Once the relative priorities of the two substituents on each of the double bond carbons has been determined, a cis orientation of the higher priority pair is designated Z, and a trans orientation is termed E. Applying these rules to the isomers of compounds A and B shown above, we assign the configuration of the 1-bromo-1-chloropropene isomer as E (Br has higher priority than Cl, and CH3 a higher priority than H). The configuration of the 1-cyclobutyl-2-ethyl-3-methyl-1-butene isomer is determined to be Z (C4H7 has higher priority than H, and the isopropyl group has higher priority than an ethyl group). The following example elaborates the priority determination for a more complex case.
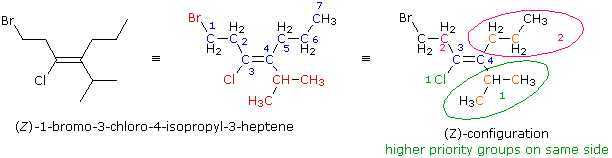
The line formula is expanded to give the structural formula in the center. The root name is heptene (the longest chain incorporating both carbons of the double bond), and the substituents (in red) are added to give the IUPAC name. In order to assign a configurational prefix the priority order of substituents at each double bond carbon must be determined. For carbon #3 the immediate substituent atoms are a chlorine and a carbon. The chlorine has a higher atomic number and therefore has higher priority (colored green and numbered 1). The more remote bromine atom does not figure in this choice. For carbon #4 the immediate substituent atoms are both carbons (colored orange). As a result, we must look at the next higher atomic number atoms in the substituent chain. These are also carbon, but the isopropyl group has two carbons (also orange) whereas the propyl group has only one. The priority order is therefore isopropyl (green) > propyl (magenta). Since the two higher priority groups (#1) are on the same side of the double bond, this configuration is (Z).
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry


