Nomenclature of Alkynes
- Page ID
- 909
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Alkynes are organic molecules made of the functional group carbon-carbon triple bonds and are written in the empirical formula of \(C_nH_{2n-2}\). They are unsaturated hydrocarbons. Like alkenes have the suffix –ene, alkynes use the ending –yne; this suffix is used when there is only one alkyne in the molecule. If a molecule contains both a double and a triple bond, the carbon chain is numbered so that the first multiple bond gets a lower number. If both bonds can be assigned the same number, the double bond takes precedence. The molecule is then named "n-en-n-yne", with the double bond root name preceding the triple bond root name (e.g. 2-hepten-4-yne).
Introduction

Here are the molecular formulas and names of the first ten carbon straight chain alkynes.
| Name | Molecular Formula |
|---|---|
| Ethyne | \(\ce{C2H2}\) |
| Propyne | \(\ce{C3H4}\) |
| 1-Butyne | \(\ce{C4H6}\) |
| 1-Pentyne | \(\ce{C5H8}\) |
| 1-Hexyne | \(\ce{C6H10}\) |
| 1-Heptyne | \(\ce{C7H12}\) |
| 1-Octyne | \(\ce{C8H14}\) |
| 1-Nonyne | \(\ce{C9H16}\) |
| 1-Decyne | \(\ce{C10H18}\) |
The more commonly used name for ethyne is acetylene, which used industrially.
Naming Alkynes
Like previously mentioned, the IUPAC rules are used for the naming of alkynes.
Rule 1
Find the longest carbon chain that includes both carbons of the triple bond.
Rule 2
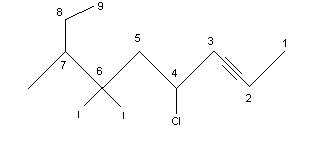
Number the longest chain starting at the end closest to the triple bond. A 1-alkyne is referred to as a terminal alkyne and alkynes at any other position are called internal alkynes. For example:
.png?revision=1&size=bestfit&width=333&height=144)
4-chloro-6-diiodo-7-methyl-2-nonyne
Rule 3
After numbering the longest chain with the lowest number assigned to the alkyne, label each of the substituents at its corresponding carbon. While writing out the name of the molecule, arrange the substituents in alphabetical order. If there are more than one of the same substituent use the prefixes di, tri, and tetra for two, three, and four substituents respectively. These prefixes are not taken into account in the alphabetical order. For example:
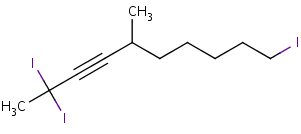
2,2,10-triiodo-5-methyl-3-decyne
If there is an alcohol present in the molecule, number the longest chain starting at the end closest to it, and follow the same rules. However, the suffix would be –ynol, because the alcohol group takes priority over the triple bond.
5- methyl-7-octyn-3-ol
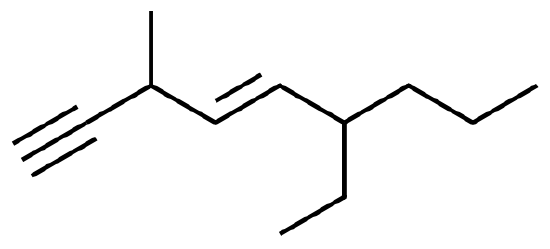
When there are two triple bonds in the molecule, find the longest carbon chain including both the triple bonds. Number the longest chain starting at the end closest to the triple bond that appears first. The suffix that would be used to name this molecule would be –diyne. For example:
4-methyl-1,5-octadiyne
Rule 4
Substituents containing a triple bond are called alkynyl. For example:
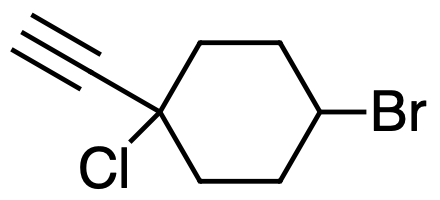
4-bromo-1-chloro-1-ethynylcyclohexane
Here is a table with a few of the alkynyl substituents:
| Molecule | |
|---|---|
| Ethynyl | -C?CH |
| 2- Propynyl | -CH2C?CH |
| 2-Butynyl | -CH3C?CH2CH3 |
Reference
- Vollhardt, Peter, and Neil E. Schore. Organic Chemistry: Structure and Function. 5th Edition. New York: W. H. Freeman & Company, 2007.
Problems
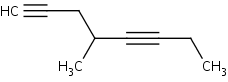
Name or draw out the following molecules:
1. 4,4-dimethyl-2-pentyne
2. 4-Penten-1-yne
3. 1-ethyl-3-dimethylnonyne
4.



.png?revision=1&size=bestfit&width=322&height=126)