8.1: E2 Reaction
- Page ID
- 359610
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)8.1.1 E2 Mechanism
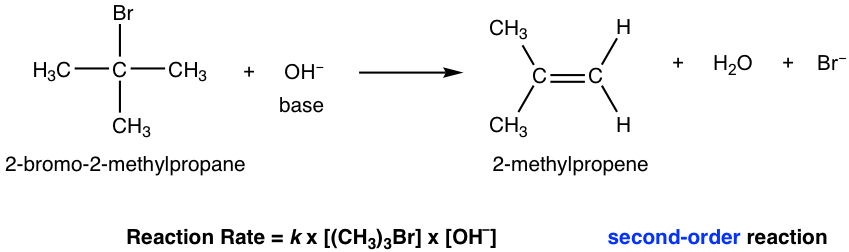
E2 mechanism is the bimolecular elimination mechanism, that the reaction rate depends on the concentration of both substrate and base. We will take the elimination reaction of 2-bromo-2-methylpropane as an example for discussion.
E2 mechanism is also a single-step concerted reaction, similar to SN2, with multiple electron pair transfers happen at the same time.
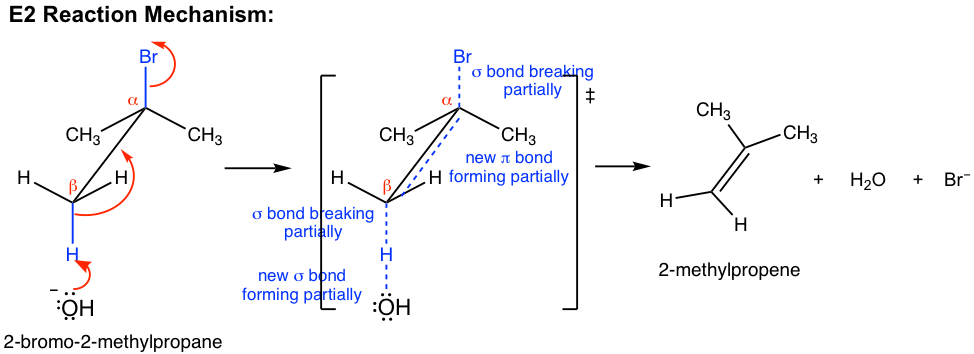
Base, OH–, uses its electron pair to attack a β-hydrogen on β-carbon, and starts to form a bond; at the same time, the β C-H sigma bond begins to move in to become the π bond of a double bond, and meanwhile Br begins to depart by taking the bonding electrons with it. A transition state is formed in the reaction process with partially breaking and partially forming bonds. At the completion of the reaction, the C=C double bond and H2O molecule are fully formed, with Br– leaves completely.
Since both the substrate (halide) and the base are involved in the single-step mechanism, E2 is the second order reaction.
8.1.2 Regioselectivity of E2 reaction: Zaitsev’s Rule vsHofmann Rule
For the reaction we talked in above section, there are three β-carbons in the substrate 2-bromo-2-methylpropane, however they are all identical, so the reaction gives only one single elimination product 2-methylpropene.
For other alkyl halides, if there are different β-carbons in the substrate, then the elimination reaction may yield more than one products. For example, the dehydrohalogenation of 2-bromo-2-methylbutane can produce two products, 2-methyl-2-butene and 2-methyl-1-butene, by following two different pathways.
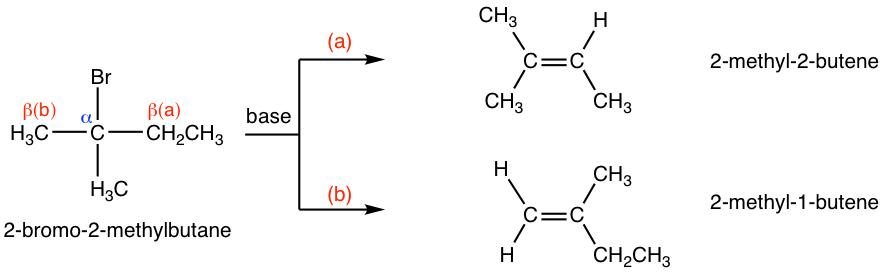
Between the two possible products, 2-methyl-2-butene is a trisubstituted alkenes, whereas 2-methyl-1-butene is monosubstituted. For alkenes, the more alkyl groups bonded on the double bond carbons, the more stable the alkene is. Generally, the relative stability of alkenes with different amount of substituents is:
tetrasubstituted > trisubstituted > disubstituted > monosubstituted > ethene
Therefore, 2-methyl-2-butene is more stable than 2-methyl-1-butene. When a small size base is used for the elimination reaction, such as OH–, CH3O–, EtO–, it turned out that the relative stability of the product is the key factor to determine the major product. As a result, 2-methyl-2-butene is the major product for above reaction.
As a general trend, when small base is applied, the elimination products can be predicted by Zaitsev’s rule, that said the more substituted alkene is obtained preferably. So the Zaitsev’s rule essentially can be explained by the higher stability of the more substituted alkenes.
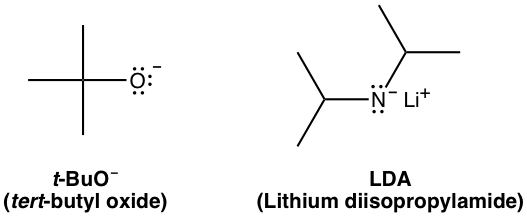
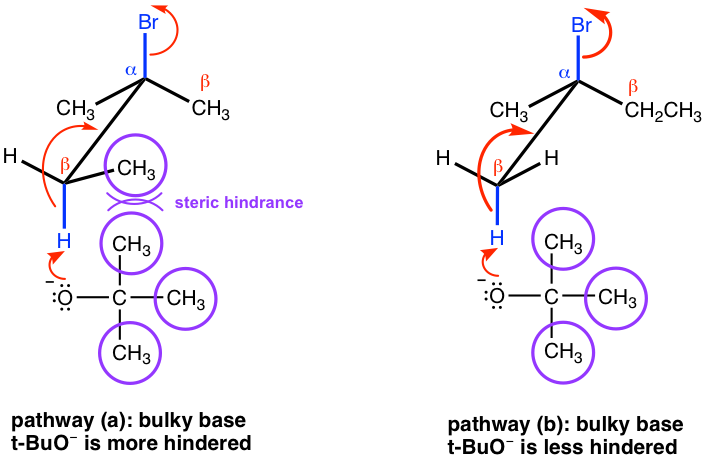
However, if a bulky base is applied in the elimination, such as t-BuOK, the reaction favors the formation of less substituted alkenes.
This is mainly because of steric hinderance. With t-BuO– attacking the β-hydrogen, it is difficult for this big bulky base to approach the hydrogens from the β-carbon that is bonded with more substituents (as shown in pathway (a) below), while the hydrogen of the methyl group is much easily to be accessed (in pathway (b) instead. When the elimination yields the less substituted alkene, it is said that it follows the Hofmann rule.
8.1.3 Stereochemistry of E2 Reaction
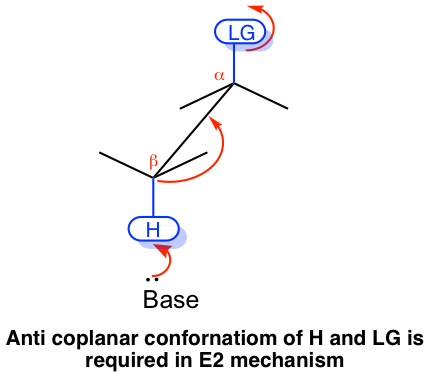
The E2 mechanism has special stereochemistry requirement to ensure it does proceed. First, the bond connected with the leaving group and the bond connected with the H must be in the same plane, to allow the proper orbital overlapping of the two carbons in the formation of π bond of the alkene product. Second, the leaving group and H must be in anti-position to each other. This is because the anti-position allows the transition state of the reaction is in the more stable staggered conformation, that helps to lower down the energy level of the transition state and speed up the reaction. Overall, E2 reaction proceeds with the leaving group and H are in anti coplanar conformation.
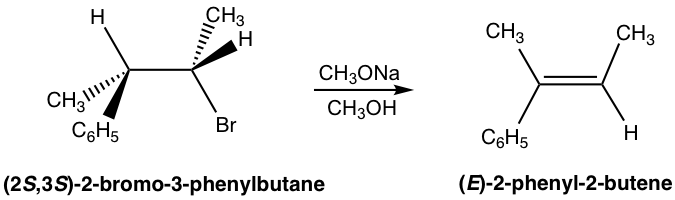
Because of the anti-coplanar conformation requirement for E2 reaction, one stereoisomer will be produced preferably over the other, and this is called stereoselectivity. For the following example, the elimination of (2S,3S)-2-bromo-3-phenylbutane produces the E isomer specifically, not the Z isomer at all. This is because when H is in anti-position to the leaving group Br, the whole compound is in staggered conformation, and the other groups retain their relative position in elimination that leads to the E isomer.
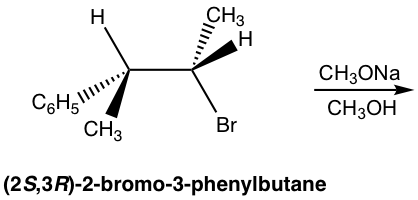
Exercises 8.1
8.1.4 Bases in E2 Reactions (Brief Summary)
The most commonly applied bases in E2 reaction are hydroxide OH–, and alkoxide RO–. Specifically, the combination of base with corresponding alcohol are used broadly, such as: CH3ONa/CH3OH, C2H5ONa/C2H5OH.
Examples of small bases: OH–, CH3O–, C2H5O–, NH2–
Examples of big bulky bases: t-BuO–, LDA (lithium diisopropylamide)