7.5: SN1 vs SN2
- Page ID
- 359607
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)7.5.1 Comparison Between SN1 and SN2 Reactions
Till now, we have finished the basic concepts about SN1 and SN2 reactions. You probably already noticed that the two type of reactions have some similarities, also quite different though. It will be very helpful to put them together for comparison. To help you get in-depth understanding of the two types of mechanism, it is highly recommended that you have a summary in your own way. The following comparison is provided here for your reference.
|
SN1 |
SN2 |
|
| Rate law |
Rate = k[electrophile] |
Rate = k[nucleophile]×[electrophile] |
| Mechanism |
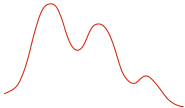
multiple steps with carbocation intermediate |
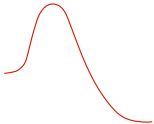
one step, concerted |
| Reaction Diagram |
|
|
| Stereochemistry |
racemization on reaction center |
inversion on reaction center |
| Electrophilic Substrate | tertiary 3° > secondary 2° > primary 1° and methyl |
primary 1° and methyl > secondary 2° > tertiary 3° |
| Nucleophile |
weak nucleophile, solvolysis |
strong nucleophile |
7.5.2 Solvent Effect on Sn1 and SN2 Reactions
Other than the factors we have talked about so far, solvent is another key factor that affect nucleophilic substitution reactions. Proper solvent is required to facilitate a certain mechanism. For some cases, picking up the appropriate solvent is the effective way to control which pathway the reaction proceed.
To understand the solvent effect, we first of all need to have more detailed discussions about solvents, then learn how to choose good solvent for a specific reaction.
Solvents can be divided into three major categories based on the structures and polarities, that is: non-polar, polar protic and polar aprotic solvents.
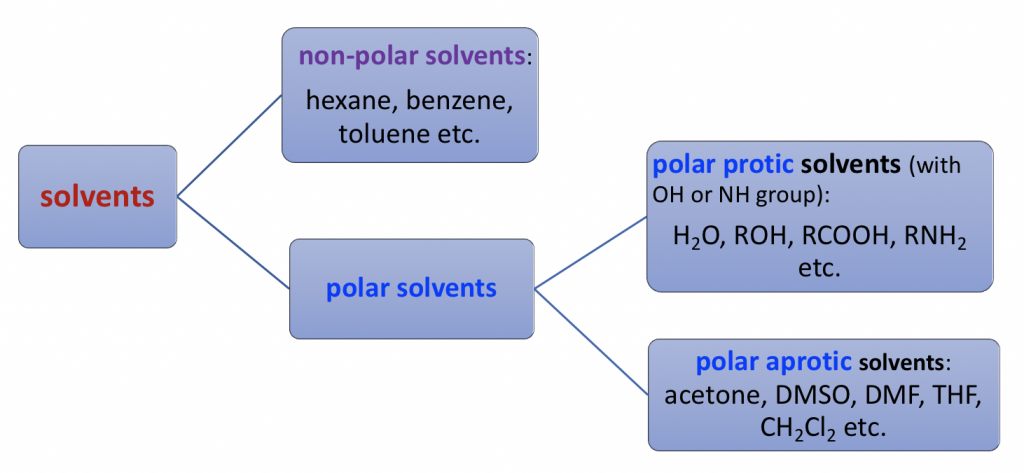
Non-polar solvents are non-polar compounds. (hexane, benzene, toluene, etc.)
Polar protic solvents are the compounds containing OH or NH group that is able to form hydrogen bonds. Polar protic solvents are highly polar because of the OH or NH group.
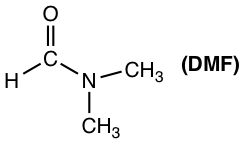
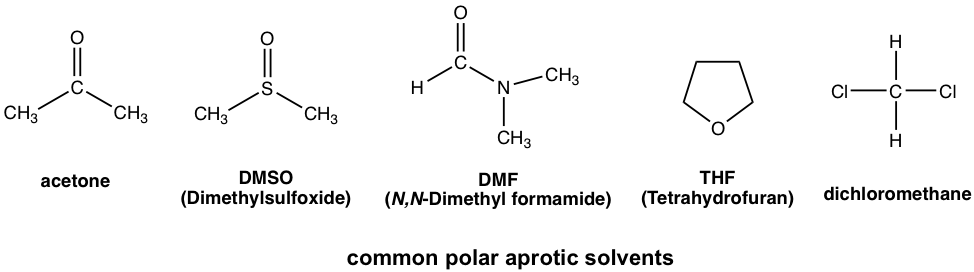
Polar aprotic solvents is a group solvents with medium range of polarity. They are polar because of polar bonds like C=O or S=O, but the polarity is not as high as OH or NH group. Typical examples of polar aprotic solvents include acetone, DMSO, DMF, THF, CH2Cl2.
The general guideline for solvents regarding nucleophilic substitution reaction is:
- SN1 reactions are favored by polar protic solvents (H2O, ROH etc), and usually are solvolysis reactions.
- SN2 reactions are favored by polar aprotic solvents (acetone, DMSO, DMF etc).
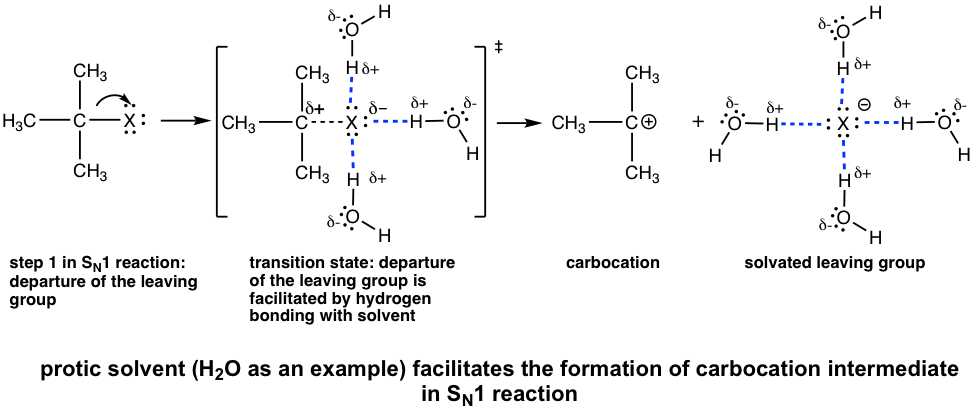
Polar Protic Solvents Favor SN1 Reactions
In SN1 reaction, the leaving group leaves and carbocation formed in the first step, that is also the rate-determining step. The polar solvent, such as water, MeOH, is able to form hydrogen bonding with the leaving group in the transition state of the first step, therefore lowering the energy of the transition state that leads to the carbocation, and speed up the rate-determining step. As a result, polar protic solvents facilitate SN1 reactions. It is very common that the polar protic solvents serve as nucleophiles as well for SN1 reactions, so usually SN1 reactions are solvolysis reactions as we learned earlier.
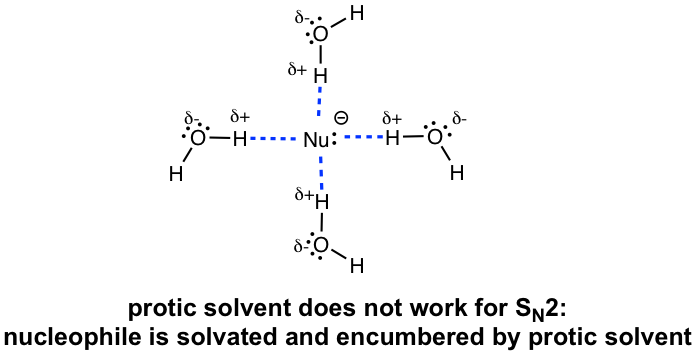
Polar Aprotic Solvents Favor SN2 Reactions
Strong nucleophiles are required in SN2 reactions, and strong nucleophile are usually negatively charged species, such as OH–, CH3O–, CN– etc. These anions must stay with cations in salt format like NaOH, CH3ONa etc. Since salts are insoluble in non-polar solvent, therefore non-polar solvents are not appropriate choices, and we need polar solvents that can dissolve the salts.
The issue for polar protic solvent is that the nucleophile anions will be surrounded by a layer of solvent molecules with hydrogen bonds, and this is called the solvation effect. The solvation effect stabilize (or encumber) the nucleophiles and hinder their reactivities in SN2 reaction. Therefore, polar protic solvents are not suitable for SN2 reactions.
As a result the polar aprotic solvents, such as acetone, DMSO etc are the best choice of SN2 reactions. They are polar enough to dissolve the salt format nucleophiles, and also not interact as strongly with anions to hinder their reactivities. The nucleophile anions still move around freely in polar aprotic solvent to act as nucleophile.
The reaction rate for a SN2 reaction in different solvents are provided in the table below, and the polar aprotic solvent DMF proved to be the best choice that speed up the reaction significantly.
|
reaction: CH3I + Cl– → CH3Cl + I– |
|
|
solvent |
relative rate |
|
CH3OH |
1 |
|
|
12.5 |
|
|
1,200,000 |
With all the knowledge about SN1, SN2 reactions and reaction conditions, we should be able to determine that whether a given reaction go with SN1 or SN2 pathway, or design a proper reaction that will produce the desired product(s). The reaction pathway predominantlydepends on the nature of the substrates (primary, secondary or tertiary), and the choice of proper reaction condition serve as a way to facilitate the process.
- Primary and methyl substrates undergo SN2 reaction predominantly.
- Tertiary substrates go with SN1 process.
- The reaction of secondary substrates mainly rely on the conditions applied. The condition include nucleophile, solvent, etc. See examples for more detailed discussions.
Exercises 7.4
Show the product(s) of the following reactions:
- (S)-2-iodobutane + CH3O–Na+ (DMSO →_
- (S)-2-iodobutane (CH3OH →_
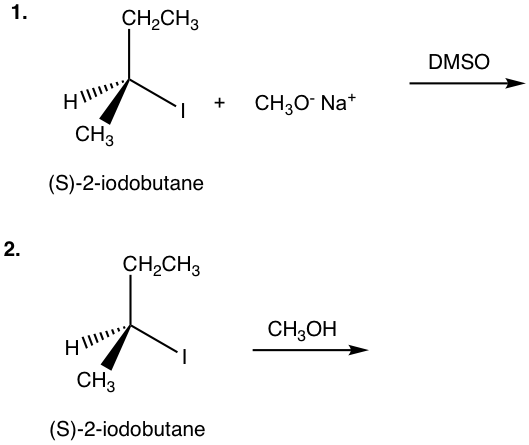
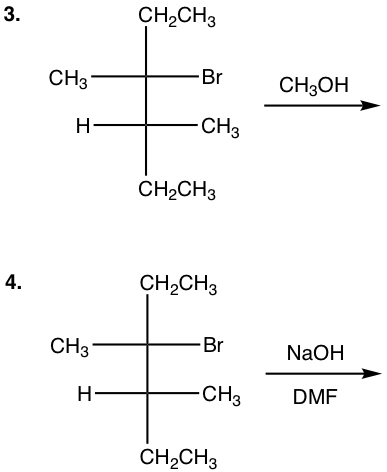
Answers to Practice Questions Chapter 7
Some practical tips for working on SN1, SN2 reactions:
- As we understand that strong nucleophiles are required for SN2 reaction, and most of the strong nucleophiles are those with negative charges, for example OH–, OR–. These nucleophiles can be either shown as anions OH–, CH3O–, C2H5O–, or in salt format like NaOH, KOH, CH3ONa, C2H5ONa in the reaction conditions. You should understand that it is the same thing. The anion format are easy to identify and also highlight the nature of these species, however since anions must stay together with counter cations as salt, the salt format show the actual chemical formula of the compound used in the reaction.
- Since polar aprotic solvent favors SN2 reactions, so any of above anions or salt can be used together with DMSO, DMF etc, such as OH–/DMSO, CH3ONa/DMF etc .
However, sometimes you may see the combination like CH3ONa/CH3OH, that is the combination of CH3O– together with its conjugate acid CH3OH. It may seems contradictory, why a strong nucleophile for SN2 combine with solvent for SN1? The reality is that CH3ONa here still act as strong nucleophile and can be used for SN2 reaction and CH3OH is the solvent for CH3ONa. The reason why CH3OH is used together as solvent is that the CH3ONa can be prepared by treating an alcohol with Na. For example:
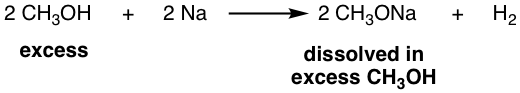
Other alcohol can also react with Na metal (or potassium metal, K) to generate the corresponding RONa.
The reaction between alcohol and NaH can be used as well.
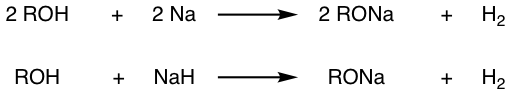 Since alcohol are in excess in the above reactions, it is also a good solvent for the resulting alkoxide, and RO–/ROH combination is used commonly together. The RO– in this combination can be used as strong nucleophile for SN2 reaction, or base in elimination reaction (Chapter 8).
Since alcohol are in excess in the above reactions, it is also a good solvent for the resulting alkoxide, and RO–/ROH combination is used commonly together. The RO– in this combination can be used as strong nucleophile for SN2 reaction, or base in elimination reaction (Chapter 8).