7.3: Other Factors that Affect SN2 Reactions
- Page ID
- 359605
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Leaving Group
When alkyl halides undergo nucleophilic substitution reactions, halogen is the leaving group. Not only halogens can be leaving group, other appropriate groups could be leaving groups as well. Generally speaking, nucleophilic substitution reaction requires good leaving group. How to determine whether a leaving group is good or not then? When leaving group departs, it takes the electron pair from the broken bondtogether it. So the good leaving group should be the one that can accommodate the electron pair very well with it, or it can be said the good leaving group should be stable with the pair of electrons.
The stability of a group with electron pair is related to the basicity of the group, since basicity means the ability of the species to share its electron pair. As a result, strong base has the high reactivity to share the electron pair, so it is not stable, and cannot be good leaving group. On the other side, weak base with low tendency to share the electron pair, is more stable base and therefore is good leaving group. So the general trend is:
The weaker the basicity of a group, the better leaving group it is .
Our knowledge in acid-base topic will be very helpful here to compare the strength between different leaving groups.
For alkyl halides, the relative reactivities as leaving group is:
(best leaving group) I–> Br–> Cl–> F– (weakest leaving group)
This order matches with the relative basicity of halide anions, I– is the weakest base and also the best leaving group.
Beside halides, other groups can be leaving groups as well. In acid-base chapter we have learned about some examples of strong organic acids, for example, tosylic acid, TsOH, etc. Since the conjugate base of strong acid is very weak bases, the conjugate bases of those acids are good choice of leaving group as well. Examples include (the leaving group is highlighted in blue color):
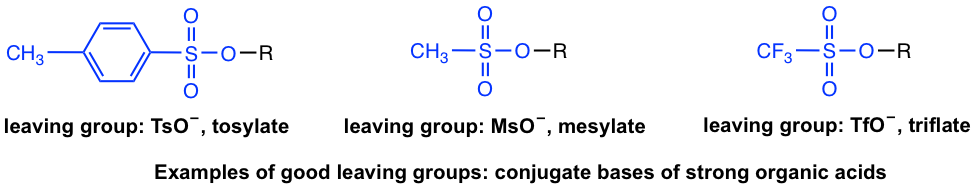
Strong bases such as OH–, RO–, NH2–, R– are therefore very poor leaving groups and cannot go with nucleophilic substitution reactions. For OH– or RO– however, upon protonation they can be converted to neutral H2O or ROH molecules, that are good leaving groups suitable to substitution. This topic will be covered in section 7.6.
Note : with the scope of leaving group expanded, the substitution reaction not only limited to alkyl halide. Any compounds with a good leaving group can undergo nucleophilic substitution.Nucleophile
For SN2 reaction, nucleophile is one of the rate-determining factors, therefore strong nucleophile helps to speed up SN2 reactions.
The relative strength of a nucleophile is called nucleophilicity. Nucleophilicity of a nucleophile is measured in terms of the relative rate of its SN2 reaction with the same substrate. Generally speaking, the nucleophilicity trend depends on several structural features of the nucleophile.
- A nucleophile with negative charge is always stronger than the corresponding neutral one. For example: OH–> H2O; RO–> ROH.
- Nucleophilicity decrease across a period. For example: NH3 > H2O; RNH2 > ROH
- Nucleophilicity increase across a group. For example:
RSH > ROH; RS–> RO–;
I– > Br– > Cl– > F– (protic solvent)
- Smaller group is better nucleophile than bulky group.
For example, t-BuO– 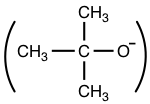 is very poor nucleophile because of its bulky size.
is very poor nucleophile because of its bulky size.
To make it more convenient for studying purpose, the commonly applied strong and weak nucleophiles are listed here:
Strong (good) nucleophile: OH–, RO– (small alkoxide), RS–(thiolate), N3–(azide), CN–(cyanide), Cl–, Br–, I–(halide), RCO2–(carboxylate), RNH2 (amine)
Weak (poor) nucleophile: ROH, H2O, t-BuO–
With the structure of nucleophiles being so diverse, SN2 reaction can be used to synthesize the compounds with a variety of functional groups, as shown here.
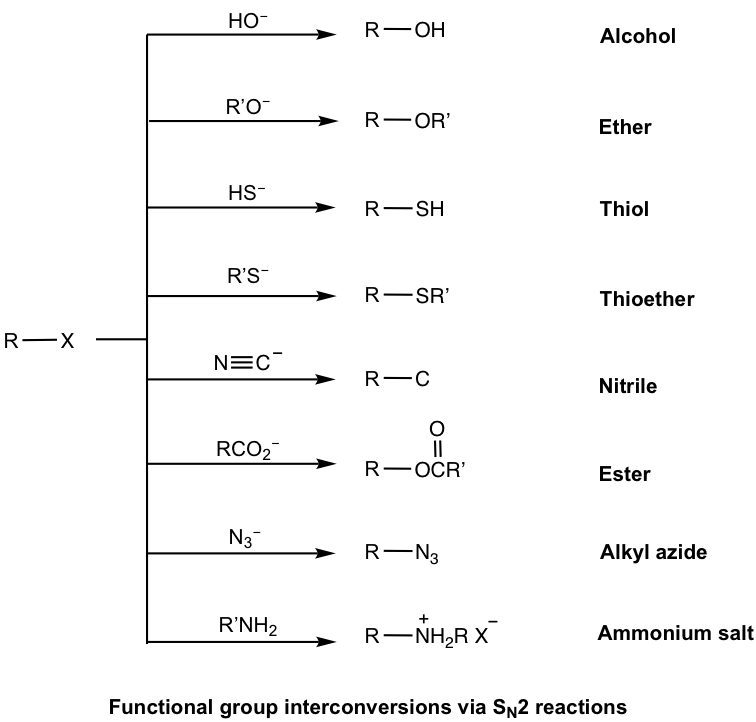
Examples
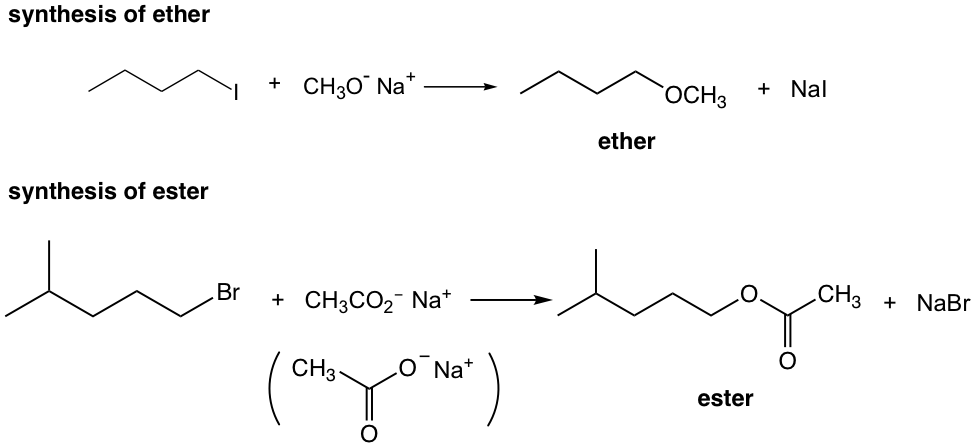
Exercises 7.2
Show reaction mechanism of the above reactions.


