7.1: Nucleophilic Substitution Reaction Overview
- Page ID
- 359603
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Let’s start with a simple substitution reaction example:
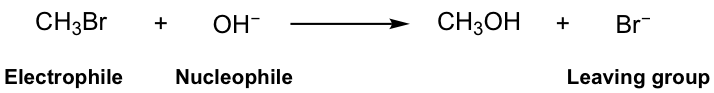
In this reaction, the Br in the reactant methylbromide (CH3Br) is replaced by the OH group, and the methanol (CH3OH) is produced as the major product, together with bromide Br-, the side product. It is easy to understand that this is a substitution reaction, because Br is substituted by OH.
Further discussions on this simple reaction require the introduction of some key terms that are critical in understanding why and how the reaction proceed in this way. These terms are electrophile, nucleophile and leaving group.
Electrophile
The reactant CH3Br is an alkyl halide. The C-X bond (X: F, Cl and Br) in alkyl halide is polar because halogen is more electronegative than carbon, and as a result carbon has a partial positive charge and halogen has a partial negative charge.
![]()
Because of the partial positive charge on carbon, the carbon atom in C-X bond is electron-deficient, and it is going to seek electron-rich reagent to connect with. Such electron-deficient species is called an electrophile (phile is the Greek suffix means “love”), means the species that loves electrons. The electron-deficient species are usually electrophiles. Other electrophile examples include positive charged ions and atom with incomplete octet, for example: H+, CH3+,BH3, BeF2, AlCl3.
For CH3Br in this reaction, it is the carbon atom that act as the electrophile, and the carbon can be called as electrophilic carbon.
The compound CH3Br that undergoes the substitution usually can be called the substrate. NucleophileThe hydroxide, OH–, is another reactant in above reaction. It is shown clearly with the Lewis structure of OH– that the oxygen atom has three lone pair electrons and is negatively charged, so it is an electron-rich species with high electron density.
![]()
An electron-rich species is called a nucleophile (“nucleo” comes from nucleus, that means positive charge), that is the reagent seeking positively charged or electron-poor species to react with. OH– is the nucleophile for above reaction. Generally, any species with the electron pair available for sharing could be nucleophile. Nucleophile can be either negatively charged (Nu:–), or neutral (Nu:), for example: OR–, H2O, ROH, NH3, RNH2, RCOO– are all possible nucleophiles.
Based on the understanding of the concepts of electrophile and nucleophile, you probably realize that a nucleophile could react with an electrophile! Yes, that is the very important and fundamental rule for organic reaction: when electron-rich nucleophile meet with electron-deficient electrophile, organic reaction would occur.
Leaving Group
To ensure the above substitution occurs, another critical factor is that the Br must leave together with the electron pairs in C-Br bond, and the bromide, Br-, is called the leaving group. The leaving group (LG) leaves with the bonding pair of electrons, and is replaced by the nucleophile in the substitution reaction. Without a proper leaving group, even nucleophile is attracted to electrophile, the substitution reaction still cannot move forward. Leaving group can be negatively charged or neutral, as we will see in detailed discussions later.
Applying the three key terms, the above substitution reaction can be summarized as: the nucleophile displaces the leaving group in a substrate, so such reaction is called nucleophilic substitution reaction. Nucleophilic substitution reaction could therefore be shown in a more general way:
![]()
Note: the nucleophile and leaving group are not necessary negatively charged, they could be neutral as mentioned earlier.
Kinetics of Nucleophilic Substitution Reaction
Kinetics is the study that concerns the rate of a chemical reaction, or how fast the reaction occurs. The reaction rate data helps to shine a light on the understanding of reaction mechanism, the step-by-step electron transfer process. Kinetic studies on nucleophilic substitution reactions indicate that there are two different rate law expressions for such reactions. For the two reactions below, reaction 1 is in second order while reaction 2 is in first order. The only reason behind the different kinetic rate is that the reactions go through different reaction mechanism.
![CH3Br + OH- = CH3OH + Br-, Reaction Rate = k times [CH3Br] times OH-](https://chem.libretexts.org/@api/deki/files/397583/Kinetics-rx1.png?revision=1)
Reaction 1 is the substitution reaction we are familiar with already. It is a second-order reaction. That means the reaction rate depends on the concentration of both substrate CH3Br and nucleophile OH–. If the concentration of CH3Br doubled, the reaction rate get doubled, and if the concentration of OH– doubled, the reaction rate doubled as well. When the concentration of both CH3Br and OH– doubled, the reaction rate increased by a factor of four.
![(CH3)3CBr + H20 = (CH3)3COH + HBr, reaction rate = k times [(CH3)3Br]](https://chem.libretexts.org/@api/deki/files/397584/Kinetics-rx2.png?revision=1)
Reaction 2 is another substitution reaction example. The substrate here is a tertiary bromide and the nucleophile is neutral water molecule. As a first-order reaction, the reaction rate depends only on the concentration of substrate (CH3)3CBr and has nothing to do with nucleophile.
The two types of reactions correspond to two types of reaction mechanism:
- The second-order reaction goes through the bimolecular reaction mechanism that is called SN2 reaction, meaning Substitution, Nucleophilic and Bimolecular.
- The first-order reaction goes through the unimolecular reaction mechanism that is called SN1 reaction, meaning Substitution, Nucleophilic and Unimolecular.
We will have detailed discussions on SN2 and SN1 mechanism respectively, and then compare the similarities and differences in between.


