3.3: pKa of Organic Acids and Application of pKa to Predict Acid-Base Reaction Outcome
- Page ID
- 359577
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)As we mentioned before, all organic compounds could be acids, because they all have hydrogen atoms that could potentially be donated. Most organic acids are weak acids with a small Ka. For example, acetic acid CH3COOH has a Ka of 1.8×10-5. Lots of other organic acids are even weaker than acetic acid, and it is this weak acidity that makes it difficult to realize that some organic compounds are actually acids.
However, this weak acidity is very important in Organic Chemistry. Since it is not that very convenient to say or to remember Ka values like 1.8×10-5, pKa is used more often in Organic Chemistry to refer to the relative acidity of different acids. The definition of pKa is:
pKa = -logKa
The smaller the pKa value, the larger the Ka, and the stronger the acidity is.
The pKa of most organic acids range between 5~60. While it is impossible to know the pKa of every organic compound, it is very useful to understand the pKa (and acidity) based on the functional groups involved, because the same functional groups usually have similar pKas. The approximate ranges of pKa values for seven major functional groups are listed in Table 3.1, which serves as a very valuable starting point for us to predict and understand the acidity of any organic molecule. The strongest organic acid listed here is carboxylic acid, with a pKa of about 5; the weakest organic acids are the alkanes with pKa values of over 50. Since approximate ranges of pKa values are listed in the table, the exact pKa value of a group varies for different compounds because of the structural differences. Fortunately however, it is usually not necessary to know the exact pKa values for most cases in organic chemistry, and the approximate range is good enough.

- Acidity is the ability of a compound to donate H+, so when we talk about the acidity (Ka and pKa) of an organic compound, it must be about a specific H atom (highlighted blue in the table). For different H atoms in the same compound, the acidity and pKa are different. As for the example of methanol:

- It is very useful to memorize the approximate ranges of pKa listed in Table 3.1.
- The acidity of the functional groups in the table decreases from top to the bottom, and the basicity of the conjugate bases in the last column increases from top to bottom, because the stronger the acid, the weaker the conjugate base is.
Predict the Outcome of Organic Acid-Base Reaction — Use pKa as Criterion
With the knowledge of acidity and pKa, we are now ready to see how to apply this information to the understanding of organic reactions from an acid-base perspective.
The following reaction is an example in Section 3.2. If you take a closer look at the reactants and products, you will find that the “product” side also contains an acid (ammonia NH3), and a base (methoxide CH3O–). Now the question is, how can we be so sure that the reaction proceeds to the “product” side as written? The question can also be asked in a different way: if equilibrium is established for the reaction mixture, which side will the position of the equilibrium predominantly favour? Left or right?

To answer that question, we will learn about a general rule for acid-base reaction: Acid-base reactions always favour the formation of the weaker acid and the weaker base. This is because the equilibrium always favours the formation of more stable products, and weaker acids and bases are more stable than stronger ones.
![HA (stronger acid) + B (stronger base) [smaller pKa] = A- (weaker base) + HB+ (weaker acid) [larger pKa]](https://chem.libretexts.org/@api/deki/files/397335/equilibrium-reaction-2.png?revision=1)
With pKa values available at hand, the relative acidity of reactants vs products can be compared by comparing their pKa values, and the reaction will proceed to the side of the acid with a larger pKa (larger pKa means smaller Ka, therefore weaker acid).
So for this reaction, the pKa check indicates that ammonia NH3 is a weaker acid than methanol CH3OH, so the reaction does proceed to the right side with CH3O– and NH3 as the major products.

Notes: Only comparing between acids is good enough for this purpose, because if CH3OH is stronger than NH3, then the conjugate base CH3O– must be weaker than the other base NH2–.
Examples
Show the products of the following reactions and predict the predominant side of the equilibrium.
Reaction 1

Reaction 2
![]()
Solutions:
Reaction 1

Reaction2
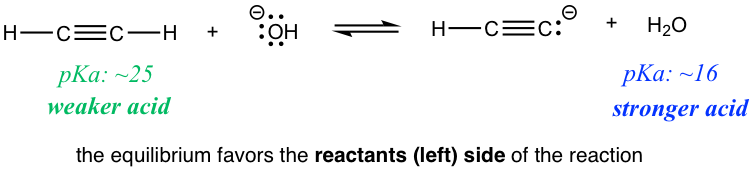
Are there any practical applications for such a prediction? Yes! Let’s compare the two reactions in the exercises above. Reaction 1 indicates that if ethyne (HC≡CH) and amide (NH2–) are mixed together, the reaction does proceed to the products side, meaning HC≡CH could be deprotonated by amide NH2–. However, if HC≡CH and hydroxide OH– are mixed together as shown in reaction 2, no reaction occurs, or we can say that HC≡CH can not be deprotonated by OH– because OH– is not strong enough! So if you are working in the lab and have the option of choosing between NH2– or OH– to deprotonate HC≡CH, you now know which one to choose.
The idea that OH– is not a strong enough base may bother you a lot, since it conflicts with the “common knowledge” that we learned in General Chemistry, where OH– is a strong base. Generally speaking, OH– is a pretty strong base; however, it is just barely not strong enough to deprotonate HC≡CH, which is a very weak acid, with a pKa of about ~25. Since HC≡CH is much weaker than the “weak acids” we learned in General Chemistry, a much stronger base, like NH2–, is required to deprotonate it.

