2.5: Degree of Unsaturation/Index of Hydrogen Deficiency
- Page ID
- 359572
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Now with lots functional groups introduced, the extent of constitutional isomers will be expanded a lot. To further explore the phenomena of constitutional isomers, we will need to understand the concept of Degree of Unsaturation (or: Index of Hydrogen Deficiency/IHD).
Let’s compare three compounds first: pentane, 1-pentene and cyclopentane

The formula for pentane is C5H12. For a compound containing 5 carbons, the maximum number of hydrogens is 12, so the structure of pentane is saturated (no more hydrogen atoms can be added in), or we can say that pentane has zero degree of unsaturation.
For 1-pentene C5H10, there are two less hydrogens than the saturated level (pentane), which means the 1-pentene has one degree of unsaturation. With a ring introduced, cyclopentane (C5H10) also has to sacrifice two hydrogens, so cyclopentane also has one degree of unsaturation. The trend is that when a double bond (essentially a π bond), or a ring, is involved in the structure, it leads to one degree of unsaturation of the compound.
| Formula |
Degree of Unsaturation/ Index of Hydrogen Deficiency (IHD)* |
Structure Unit Involved |
| CnH2n+2 |
0 |
chain alkane only |
| CnH2n |
1 |
1 double bond
or 1 ring |
| CnH2n-2 |
2 |
2 double bonds
or 2 rings or 1 double bond plus 1 ring or 1 triple bond |
Table 2.5 Summary of degree of unsaturation/IHD vs structure unit involved
The degree of unsaturation could be accumulated, and Table 2.5 summarizes the situations up to two degrees. As we can see, adding 1 ring or 1 π bond contributes to one degree of unsaturation. Therefore, the essential meaning of degree of unsaturation is the “number of rings plus π bonds” in a structure.
If the structure of a compound is available to us, the total degrees of unsaturation can simply be counted through inspecting the structure.
Example:


If the formula of a compound is given, we can also calculate the degree of unsaturation by comparing the number of hydrogens vs the saturated level, by using the equation:
 (n: number of carbons; X = number of H + number of Halogen – number of N)
(n: number of carbons; X = number of H + number of Halogen – number of N)
This is a general equation that accounts for the presence of heteroatoms as well. Please note that oxygen atoms are ignored in this calculation.
For example, for a compound with a formula given as C4H7NO, it is calculated that the degree of unsaturation is 2 for this compound:
Now we are ready to solve constitutional isomer questions with the application of degrees of unsaturation. Usually, the formula information is available to us for such questions, and we will need to build constitutional isomers based on the given formula together with other requirements. To solve this type of question, it is very helpful to do it strategically by following certain steps:
- Calculate the degree of unsaturation based on the given formula.
- With the value of this specific unsaturation degree, how many double bonds or rings might be included in the structure?
- Combine your knowledge of functional groups with the degree of unsaturation, as well with certain atoms included in the formula, to see what functional group(s) may be possible.
- Build constitutional isomers according to the above information (separate the isomers by different functional group).
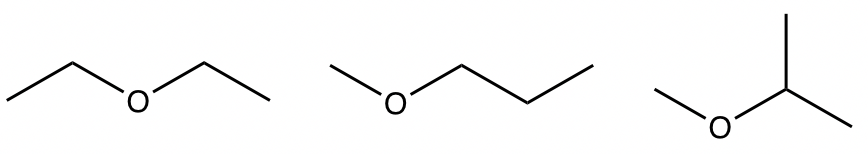
Examples: Draw and name all the constitutional isomers with the molecular formula C4H10O.
Approach: Answering the following questions lead you to the solution.
- What is the degree of unsaturation for the formula C4H10O? 0
- How many double bonds, or rings, could be involved? none
- What are the possible functional groups that matche with that degree of unsaturation, and include one oxygen atom? alcohol or ether
- With these hints, we can try to “build” the constitutional isomers for each functional group separately. total seven structures
Solutions:
alcohols:

ethers:
Exercises 2.2
Draw all the constitutional isomers that include a C=O bond with formula the C5H10O.


