2.3: Functional Groups
- Page ID
- 359570
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
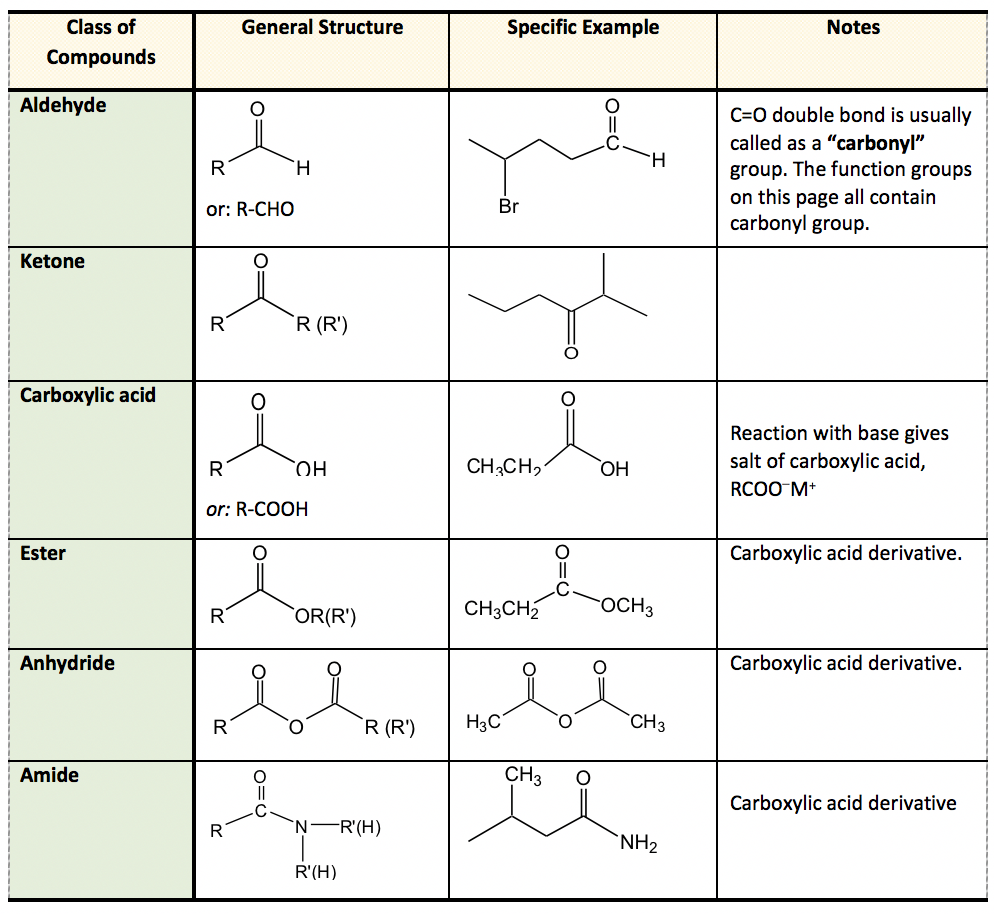
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Functional groups are the most reactive parts in organic compounds, and determine the major properties of compounds. The summary of common functional groups is included in Table 2.2. Knowing the functional groups well is one of the fundamental skills required for this course. It is required in order for students to quickly identify and name the functional groups included in molecules, as well as to understand, interpret and draw the specific structure of each functional group clearly. The IUPAC naming of compounds containing a couple of functional groups is required as well.

Alkene and alkynes are hydrocarbon functional groups; the π bond in multiple bonds accounts for the reactivity of alkenes and alkynes.
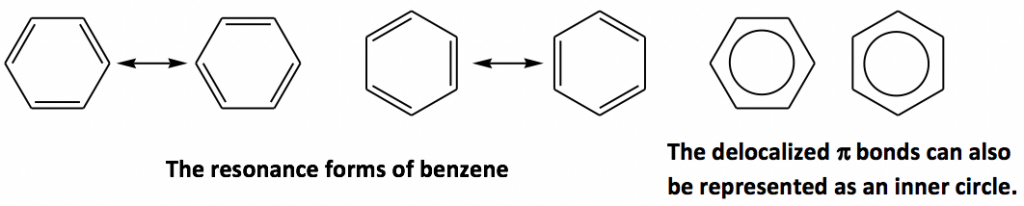
Benzene rings (C6H6) are a special type of hydrocarbon. Historically, because of the special aroma (sweet smelling) that benzene and its derivatives release, they are called aromatic compounds. The structure of benzene can be represented as three C=C double bonds alternate with single bonds, however, the actual structure of benzene has nothing to do with alkenes. Detailed discussions on the structure of benzene, which is a big conjugation system, and the chemistry definitions of aromatic/aromaticity will be a topic of Organic Chemistry II. Benzene rings can be shown with any of the following structure drawings.

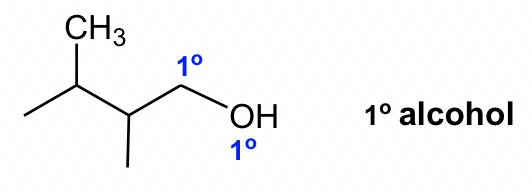
Alcohol is a functional group that you probably are familiar with. In organic chemistry, the term alcohol refers to a compound containing the OH (hydroxy) group. Depending on the position of the OH group, alcohols can also be categorized as primary (1°), secondary (2°) or tertiary (3°).
Another functional group that contains the oxygen atom in single bonds is ether. In ether, the O atom connects with two carbon-containing R groups through two C-O σ bonds. For compounds with ether as the only functional group, it is usually named with the common name “alkyl alkyl ether”. When the two alkyl groups are the same, they can be combined as “dialkyl”.
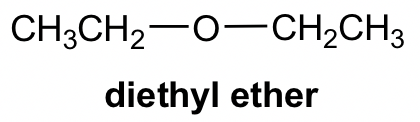
Ether can be in cyclic structure as well. It may not be that intuitive to recognize the following structure as ether, and labelling the carbon atom will be helpful for identification.
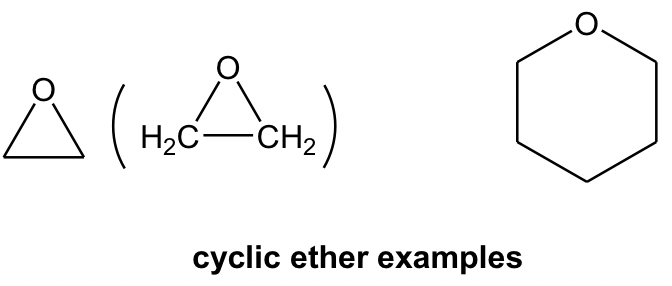
Both nitrile and nitro groups contain nitrogen atom, and it might be easy to get them mixed up. Nitrile has a C≡N triple bond, and therefore can only be at the end of a structure, while nitro (NO2) can be in any position on the carbon chain or ring.
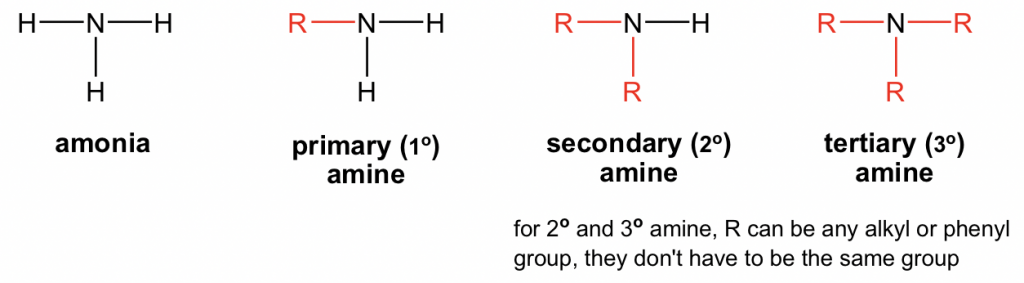
Amine is the organic derivative of ammonia, NH3. When the hydrogen atom(s) in NH3 is replaced with R groups, it produces amine. The amine can be primary (1°), secondary (2°) or tertiary (3°) depending on how many R groups are connected with nitrogen. The amines can also be named with common names.
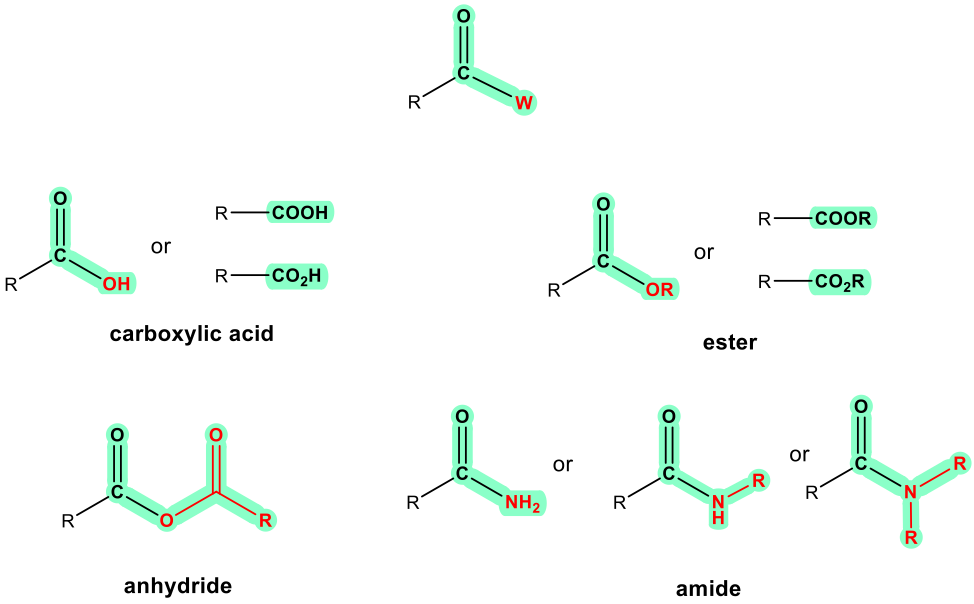
For the functional groups on the 2nd part of Table 2.2, they all have a common structural unit of a carbonyl group C=O; the different structure of “W” in the general formula determines the nature of the functional group. It is usually more challenging to identify and draw these functional groups correctly, because they are kind of similar. More practice is needed.
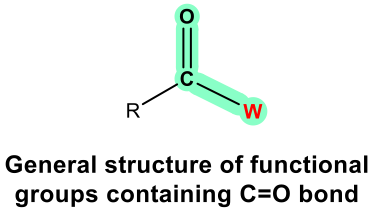
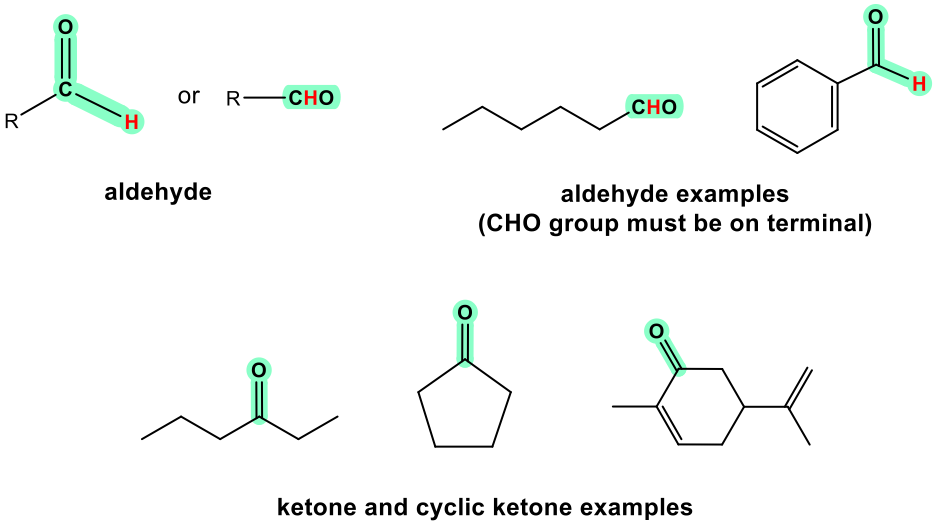
Aldehyde and ketone are similar in terms of their structures and properties. Aldehyde can be regarded as a special case of ketone since “H” can be regarded as an R with zero carbon. Because H has to be connected on one side of the C=O group in aldehyde, aldehyde can only be at the end of a structure. Ketone, on the other hand, must be in the middle position to ensure both sides of the C=O groups are connected with R groups. Ketone can also be in a cyclic structure.
The last four functional groups are related in terms of structures and chemical properties. When an OH group is connected with C=O, the whole COOH is called a carboxylic acid functional group. The other three, ester, anhydride and amide, are all derivatives of carboxylic acid, meaning they can be prepared with carboxylic acid as the starting material. For these three functional groups, it is important to remember that the “W” part has to be considered together with the C=O, and overall it determines the functional group correctly. For example, the COOR is ester; it can not be recognized as a “ketone” plus an “ether”.