1.1: Chemical Bonding
- Page ID
- 359561
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)To summarize simply, a chemical bond is the attractive force holding atoms or ions together. Such attractive interaction leads to a more stable state for the whole system comparing to individual atoms.
Valence electrons play a fundamental role in chemical bonding. In the electron configuration of an atom, the outermost shell is called the valence shell, and the electrons in the valence shell (outermost shell) are known as valence electrons. Take the carbon atom for example: the electron configuration of carbon is 1s22s22p2. The outermost shell is the 2nd principal shell, so there are 4 valence electrons in carbon. Valence electrons are the electrons that are the furthest away from the nucleus, and thus experience the least attraction from the nucleus and therefore are most reactive. They play the most important role in chemical bonding.
Exercises 1.1
Determine the number of valence electrons for following elements: B, N, O, Cl, Mg.
Answers to Practice Questions Chapter 1
Ionic Bond and Covalent Bond
There are two major types of chemical bonding: ionic bonds and covalent bonds. An ionic bond is a bond that results from the electrostatic attraction (force) between ions of opposite charges. Ionic bonds apply to ionic compound, such as sodium chloride (NaCl).
In simple ionic compounds, the metal element loses valence electron(s) to form the cation and the non-metal element gains electron(s) to form the anion. With the proper number of electron(s) lost or gained, both the cation and the anion achieve a full outer shell that contains eight electrons, as in the following examples of Na+, Ca2, Cl–and O2–. According to Lewis’s Theory, an atom is most stable if its outer shell is filled or contains eight electrons. This is also called the octet rule.
Na (atom) → Na+ + e– Ca (atom) → Ca2++ 2e–
Cl (atom) + e–→ Cl– O (atom) + 2e– → O2-
A covalent bond is a bond formed through the sharing of electron pairs between the two bonding atoms. The shared electron pairs are mutually attracted by the nuclei of both atoms. By sharing the electron pairs, both atoms also gain a filled outer shell, or an octet. Almost all of the bonds involved in organic compounds are covalent bonds.
Covalent bond can be non-polar or polar.
For covalent bonds formed between two identical atoms, the electron pairs are shared equally between the two nuclei. Electron density is distributed evenly through the bond, making the bond a non-polar bond. Examples include all homonuclear molecules, such as H-H, Cl-Cl, O=O, N≡N.
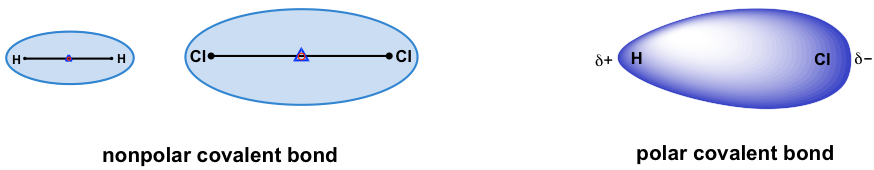
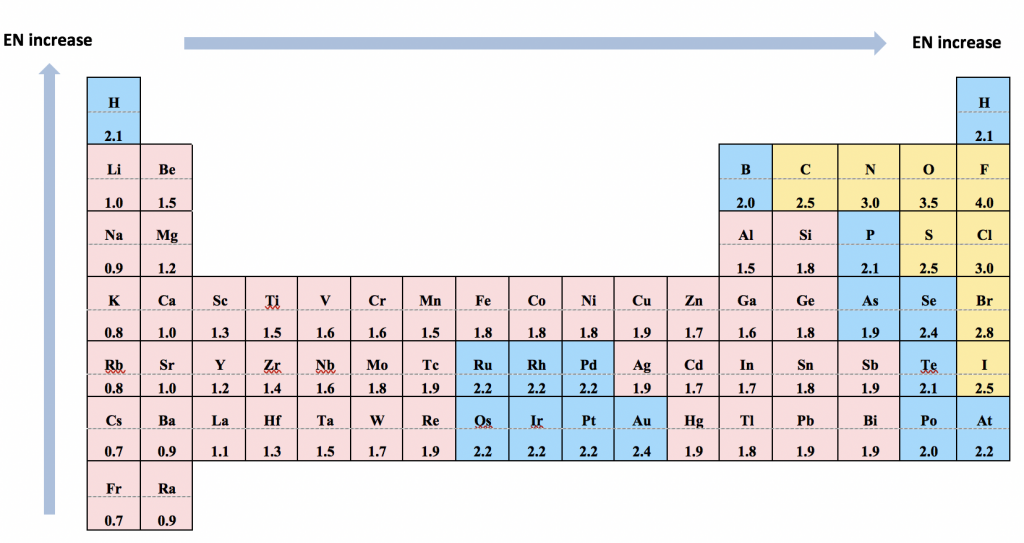
For heteronuclear bonds (the bond formed between two different atoms), the electron pairs are not shared evenly, and the bond is polar. The electron pairs are more attracted to the atom that has the stronger ability to pull the electron pairs towards itself. This ability is measured with electronegativity. The relative values of electronegativity (EN) are listed using the scale devised by Linus Pauling, as summarized in the following table:
Notes about electronegativity values for Organic Chemistry purposes:
- It is much more important to know the trend of electronegativity than to memorize the values. The trend is that EN values decrease along the group from top to bottom and increase along the period from left to right (the trend mainly works for Main Group elements, not transition metal elements).
- It is very useful (although not mandatory) to know the EN values of a few select elements: F (4.0, highest), O (3.5), N (3.0), C (2.5) and H (2.1).
- The EN of C (2.5) and H (2.1) is rather close, which makes the C-H bond (the bond involved in all organic compounds) technically non-polar.
With the introduction to the concept of electronegativity, bond polarity can be represented with the electronegativity difference between the two bonding atoms, which is known as ΔEN. For non-polar bonds, ΔEN equals to zero, and for polar bonds, ΔEN is not zero. The greater the ΔEN, the more polar the bond is.
Exercises 1.2
- Identify the following bonds as “polar” or “non-polar”: C-C, C-H, B-F, O-O, C=N
- Rank the following bonds in order of increasing bonding polarity: C—S, C—O, C—F (referring to the trend of EN, you do not need to use the exact EN values).
Answers to Practice Questions Chapter 1
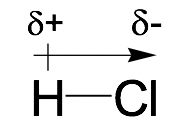
Because of the electronegativity difference, the atom with the higher EN attracts the shared electron pairs more strongly, therefore bearing a slightly negative charge (δ-). The other atom with a lower EN bears a slightly positive charge (δ+). The direction of the bond polarity can be indicated with an arrow, with the head of the arrow pointing to the negative end and a short perpendicular line near the tail of the arrow marking the positive end. The following example of an H-Cl molecule indicates how to show the bond polarity and partial charges of the polar bond.