20.2: Starting with an Alkane, Provide a Synthesis for a Molecule That Has a Functional Group Z, Where Z is a Nucleophile
- Page ID
- 216852
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)First thing to remember is that in nucleophilic reactions, the nucleophile replaces the leaving group unchanged only if it originally carried a negative charge. A neutral nucleophile, typically water or an alcohol, will lose a proton in the process of replacing the leaving group. Therefore, it is the conjugate base of such nucleophile that actually replaces the leaving group.
Sn2 reaction with a negatively charged nucleophile
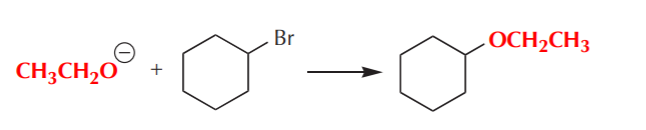
Sn1 reaction with a neutral nucleophile (most frequently H2O or ROH)

At this level we are acquainted with only one major type of leaving groups, which are the halogens (Cl, Br, and I). So if we’re supposed to start the synthesis from an alkane, we must first fit it with a good leaving group, namely a halogen. Once we have a leaving group in place we can bring in a number of different nucleophiles to replace it, depending on which functional group we’re trying to obtain at the end.
EXAMPLE 1. From propane, make 2-propanol (isopropanol). In other words, how can the following transformation be accomplished?
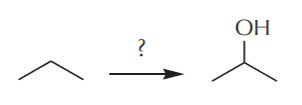
Obviously we don’t have the tools to do this in one step. So we’ll need at least two steps to do it. A retrosynthetic analysis starts from the target product and works backwards step by step until it arrives at the desired starting material. In a retrosynthesis we start from the end and worry only about the step we’re working on, momentarily forgetting the rest of the requirements. In this case, we notice that the target product is an alcohol, and alcohols contain the OH group, which can be a nucleophile. To use a nucleophile we must first have a molecule with a good leaving group (halogen). Therefore the last step in the synthesis can look something like this:

Having solved the last step, now we worry about how we’re going to obtain the bromide (isopropyl bromide). In a flash of insight, we recall that in a previous part of the course we learned how to make bromides from alkanes (free radical halogenation reaction – bingo!). Feverishly, we try to recall the conditions that led to it, and suddenly we recall that all we need is an alkane and bromine in the presence of light. We desperately scramble for a pencil to jot that down before we forget it and come up with the following:
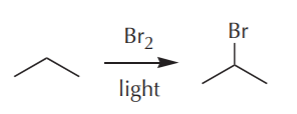
Not bad, we pat ourselves on the back. Now we’re through with the second to the last step of the synthesis. With a rush of adrenaline now pumping through our veins, we now ask what the next step would be. How can we obtain propane from...? Wait a minute! We’re supposed to start the synthesis with propane. Does that mean we’re done? You betcha! The complete synthesis would then look something like this:
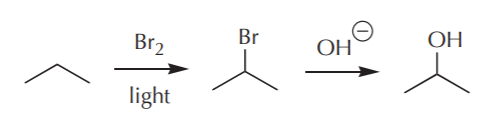
or
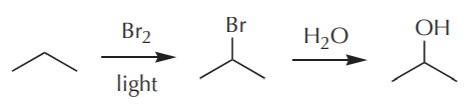
Notice that a synthetic sequence does not show all the substances that are actually present in the reaction medium, especially not the inorganic ones such as HBr. For the sake of clarity, the sequence shows only those materials of direct relevance to the task at hand, namely those organic substances whose fate we’re interested in following due to their relationship to the end product.
Also notice that a synthetic problem typically has many solutions. For example, in the synthesis above we could’ve used chlorine as the leaving group instead of bromine. We can use water as the nucleophile or OH ion. These are all equally effective solutions. The final choice is therefore frequently based on factors other then those directly related to the chemistry, for example price and availability of the substances required, their toxicity, etc.
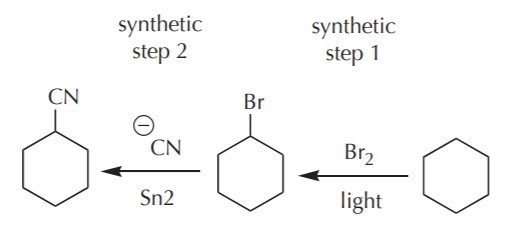
EXAMPLE 2. From cyclohexane prepare cyclohexane nitrile:
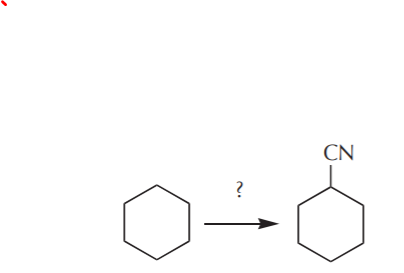
Nothing can stop us now. We immediately realize that CN is one of the nucleophiles we learned about in the chapter on nucleophilic substitutions. After a quick retrosynthetic analysis we whip out the following scheme (shown in reverse because we’re working backwards, remember?):