3.7: Polarity
- Page ID
- 214222
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)ELECTRONIC DISTRIBUTION AND BOND POLARITY
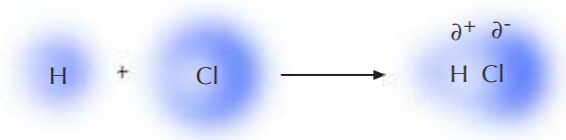
As we already learned, the atoms engaged in covalent bonding share electrons in order to fulfill the octet rule. However, this electron sharing can take place on an equal or unequal basis. If the atoms involved in covalent bonding are of equal electronegativities (which occurs only if they are the same atoms), then sharing takes place on an equal basis and there is no bias in the amount of time the bonding electrons spend around each atom. The hydrogen molecule (H2) shown below is an example of this. The electronic cloud surrounding the two atoms is highly symmetrical, and the H-H bond is said to be nonpolar.

Now consider the case of hydrogen chloride, H-Cl. Hydrogen and chlorine are engaged in covalent bonding, but the electronegativity of chlorine is higher than that of hydrogen. The greater tendency of chlorine to attract electrons results in unequal sharing between the two atoms. The bonding electrons spend more time around chlorine than around hydrogen. They are still being shared, but chlorine behaves as if it carried a negative charge, and hydrogen behaves as if it carried a positive charge. These charges are not full charges as is the case in ionic molecules. In covalent molecules they are referred to as partial charges, or poles, because they are analogous of the poles of a magnet. The positive pole is indicated by ∂ +, and the negative pole by ∂ -. The two together constitute a dipole, and the bond in question is said to be polar.
A polar bond is sometimes represented as a vector, with an arrow pointing in the direction of the more electronegative atom. The following are valid representations for polar bonds.
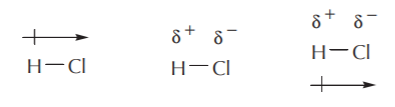
Electronegativity is the tendency of an atom to attract bonding electrons. Since the difference in electronegativity between two bonding atoms can be zero or very large, there is a polarity continuum, ranging from nonpolar to highly polar bonds. In an extreme case where the difference in electronegativity is vary large, the bond ceases to be covalent and becomes ionic.
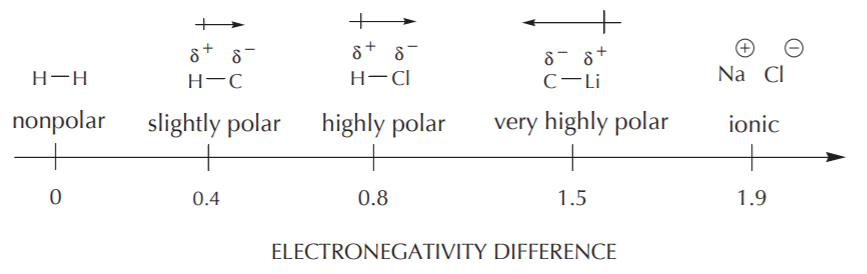
Bond polarity is measured by the dipole moment. This parameter is reported in Debye units (D). General Chemistry textbooks typically contain tables of dipole moments for different types of bonds. For example, the dipole moment for the C-H bond is 0.3 D, whereas that for the H-Cl bond is 1.09 D.
POLARITY IN ORGANIC MOLECULES
Every covalent bond is either polar or nonpolar. When all the dipoles for all the covalent bonds that make up a molecule are added together as vectors, the result is the net dipole moment of the entire molecule. When its value is zero, the molecule is said to be nonpolar, otherwise it’s said to be polar. Obviously, it is possible to have nonpolar molecules made up of polar bonds, as long as the corresponding dipoles add up to zero. Some examples are shown below. Refer to chapter 2 in your textbook for a more comprehensive discussion of polarity and dipoles.
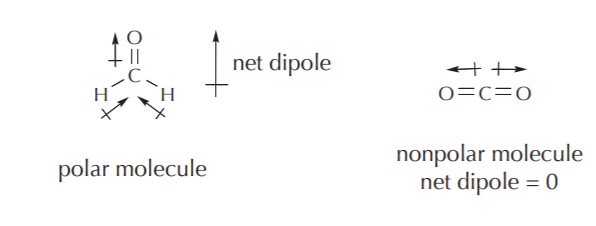
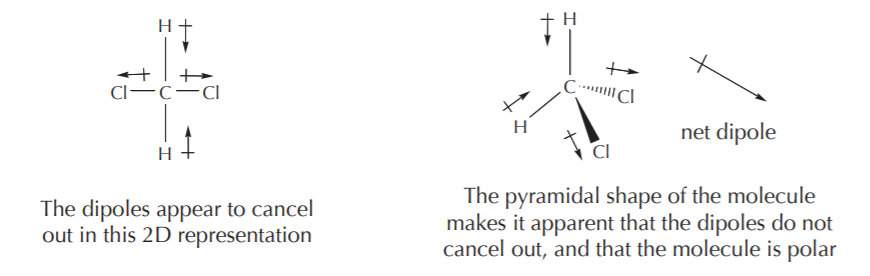
One must be careful in deciding whether a molecule is polar or nonpolar based purely on a two-dimensional representation. Molecules are three-dimensional, and direction is as important as magnitude when it comes to adding vectors. For example, a two-dimensional representation of the methylene chloride molecule (CH2Cl2) shown below might lead to the erroneous conclusion that it is nonpolar when in fact it is polar.
Many organic molecules are made up of long hydrocarbon chains with many C-H bonds. Since the difference in electronegativity between carbon and hydrogen is very small, the C-H bond has a very small dipole moment, and hydrocarbons are for the most part considered nonpolar molecules. However, the introduction of a relatively polar bond in such structures dominates the entire molecule, rendering it polar.

POLARITY AND PHYSICAL PROPERTIES
The polarity of molecules affects their physical properties. As a rule of thumb and other factors being similar, the higher the polarity of the molecule, the higher the value of properties such as melting and boiling point. The solubility of molecules in solvents is also largely determined by polarity. The rule “like dissolves like” makes reference to the fact that polar molecules dissolve better in polar solvents, and nonpolar molecules dissolve better in nonpolar solvents. Water and oil don’t mix because water is highly polar and oil is largely made up of hydrocarbon chains, which are nonpolar. Conversely, water and alcohol do mix because they are both of very similar polarities. For a more comprehensive discussion refer to chapter 2 of your textbook.


