16.3: Summary of Alkene Reactions
- Page ID
- 216683
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Alkenes are primarily prepared by elimination reactions of molecules that contain good leaving groups attached to sp3 carbons. Examples of such reactions are dehydrohalogenations with strong base, and acid-catalyzed dehydrations of alcohols. The opposite of an elimination is an addition reaction. In an addition reaction an alkene adds elements to each of the carbons involved in the π-bond, resulting in formation of sp3 carbons from sp2 carbons. This is one of the most important types of reactions that alkenes undergo.
Another important type of reaction involving alkenes is oxidative cleavage. In such reactions the carbon-carbon π-bond is completely broken by the action of an oxidizing agent, resulting in more oxidized forms of carbon, such as aldehydes, ketones, and carboxylic acids. All these reactions are covered in chapter 8 of the Wade text, 4th ed.
The following is a summary of the most important representative types of these reactions. For full details, please refer to the textbook.
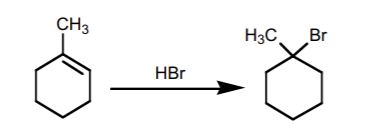
1. ADDITION OF HBr, HCl, and HI.
The addition of these substances to an alkene proceeds by an ionic mechanism, with formation of the most stable carbocation. Therefore, it follows Markovnikov’s Rule.
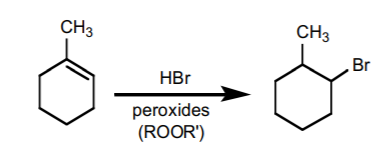
An Anti-Markovnikov variation requires the presence of peroxides as free radical initiators, and can only be performed with HBr. The mechanism proceeds with formation of the most stable free radical, which results in formation of the Anti-Markovnikov product.
THE NEXT THREE REACTIONS ADD THE ELEMENTS OF WATER (H/OH) ACROSS THE DOUBLE BOND
2. ACID-CATALYZED ADDITION OF WATER.
Produces Markovnikov alcohols, it is an equilibrium reaction, and proceeds with formation of carbocations in the rate-determining step.

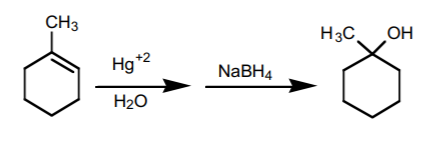
3. OXYMERCURATION-DEMERCURATION SEQUENCE.
This sequence consists of two steps. It is the most effective way to prepare Markovnikov alcohols from alkenes.
4. HYDROBORATION-OXIDATION SEQUENCE.
This sequence also consists of two steps. It is the most effective way to prepare anti-Markovnikov alcohols from alkenes. The components of water (H/OH) add to the π-bond with syn-stereochemistry. If chiral centers result from this reaction, the product is obtained as an enantiomeric mixture.
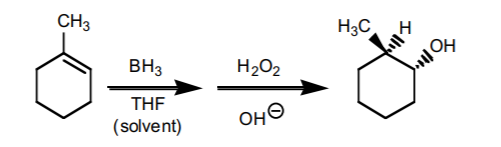
5. HALOGEN ADDITION.
This reaction is performed most frequently with bromine and chlorine. It adds a halogen atom to each of the two carbons comprising the π-bond with anti-stereochemistry. If chiral centers result from this reaction, the product is obtained as an enantiomeric mixture.
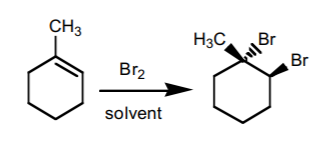
6. HALOGEN ADDITION IN THE PRESENCE OF WATER
(halohydrin formation). Produces Markovnikov alcohols with a neighboring halogen. The OH and the halogen add with anti -stereochemistry. If chiral centers result from this reaction, the product is obtained as an enantiomeric mixture.
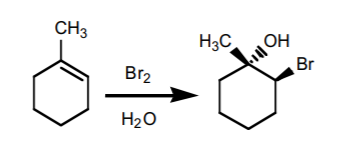
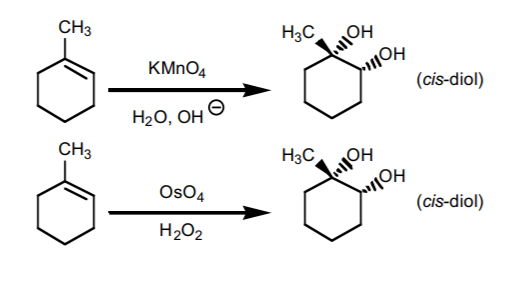
THE NEXT TWO REACTIONS ADD TWO HYDROXYL GROUPS (OH) ACROSS THE DOUBLE BOND
7. SYN HYDROXYLATION.
Adds two hydroxyl groups accross the π-bond with syn-stereochemistry. It can be performed with two reagent mixtures: pot assium permanganate in basic medium, or osmium tetroxide in the presence of hydrogen peroxide. If chiral centers result from this reaction, the product is obtained as an enantiomeric mixture.
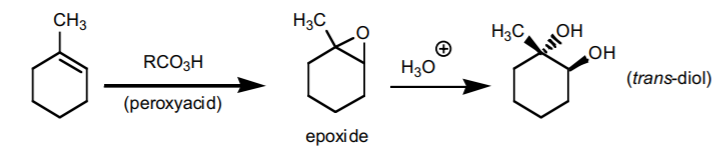
8. ANTI-HYDROXYLATION SEQUENCE.
Adds two hydroxyl groups accross the π-bond with anti-stereochemistry. It proceeds in two steps via an epoxide (three-membered ring ether). If chiral centers result from this reaction, the product is obtained as an enantiomeric mixture.
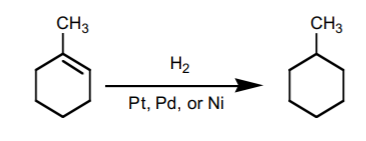
9. CATALYTIC HYDROGENATION.
Adds hydrogen accross the π-bond with syn-stereochemistry. It basically transforms an akene into an alkane.
MAIN OXIDATIVE CLEAVAGE REACTIONS
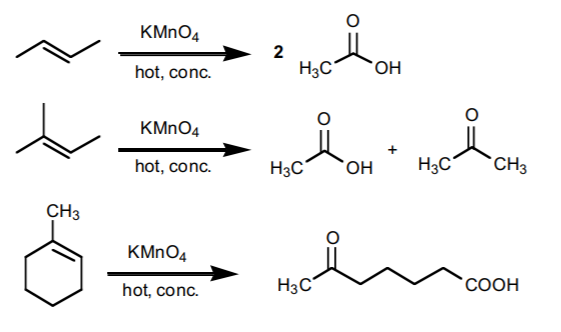
1. CLEAVAGE WITH HOT, CONCENTRATED POTASSIUM PERMANGANATE
yields ketones and/or carboxylic acids, depending on whether the carbon in question is in the middle of a chain or at the end of a chain in the product.
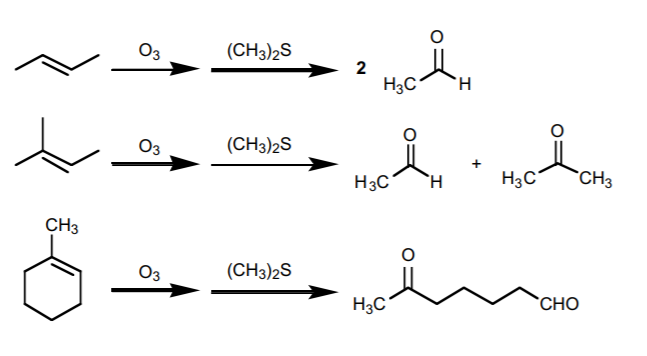
2. OZONOLYSIS.
Similar to the above, but it yields only carbonyl compounds (aldehydes or ketones). Ozone is required for the reaction, but the other reagent may vary from one context to another.