General Steroid Structure
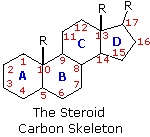
The important class of lipids called steroids are actually metabolic derivatives of terpenes, but they are customarily treated as a separate group. Steroids may be recognized by their tetracyclic skeleton, consisting of three fused six-membered and one five-membered ring, as shown in the diagram below. The four rings are designated A, B, C & D as noted, and the peculiar numbering of the ring carbon atoms (shown in red) is the result of an earlier misassignment of the structure. The substituents designated by R are often alkyl groups, but may also have functionality. The R group at the A:B ring fusion is most commonly methyl or hydrogen, that at the C:D fusion is usually methyl. The substituent at C-17 varies considerably, and is usually larger than methyl. The most common locations of functional groups are C-3, C-4, C-7, C-11, C-12 & C-17. Ring A is sometimes aromatic. Since a number of tetracyclic triterpenes also have this tetracyclic structure, it cannot be considered a unique identifier.
Common Steroids
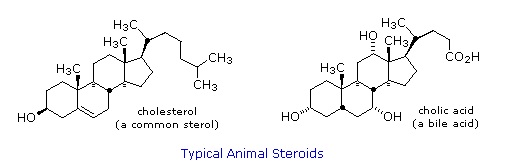
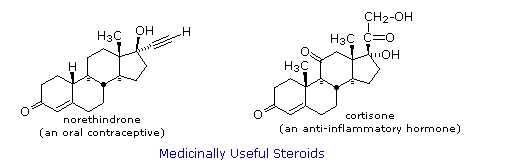
Steroids are widely distributed in animals, where they are associated with a number of physiological processes. Examples of some important steroids are shown in the following diagram. Norethindrone is a synthetic steroid, all the other examples occur naturally.
The generic steroid structure drawn above has seven chiral stereocenters (carbons 5, 8, 9, 10, 13, 14 & 17), which means that it may have as many as 128 stereoisomers. With the exception of C-5, natural steroids generally have a single common configuration.
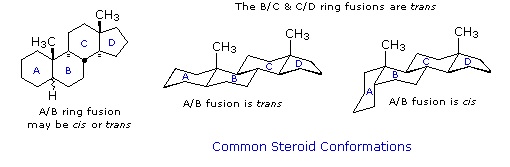
Chemical studies of the steroids were very important to our present understanding of the configurations and conformations of six-membered rings. Substituent groups at different sites on the tetracyclic skeleton will have axial or equatorial orientations that are fixed because of the rigid structure of the trans-fused rings. This fixed orientation influences chemical reactivity, largely due to the greater steric hindrance of axial groups versus their equatorial isomers. Thus an equatorial hydroxyl group is esterified more rapidly than its axial isomer.
Decalin as a model system
It is instructive to examine a simple bicyclic system as a model for the fused rings of the steroid molecule. Decalin, short for decahydronaphthalene, exists as cis and trans isomers at the ring fusion carbon atoms. Planar representations of these isomers are drawn at the top of the following diagram, with corresponding conformational formulas displayed underneath. The numbering shown for the ring carbons follows IUPAC rules, and is different from the unusual numbering used for steroids. For purposes of discussion, the left ring is labeled A (colored blue) and the right ring B (colored red). In the conformational drawings the ring fusion and the angular hydrogens are black.
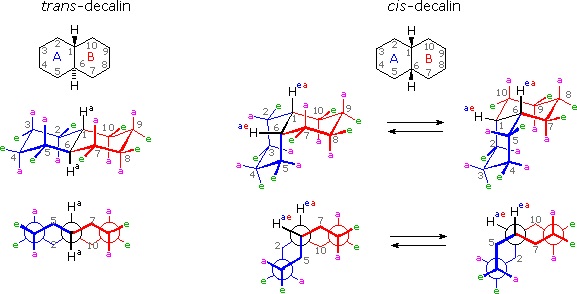
The trans-isomer is the easiest to describe because the fusion of the A & B rings creates a rigid, roughly planar, structure made up of two chair conformations. Each chair is fused to the other by equatorial bonds, leaving the angular hydrogens (Ha) axial to both rings. Note that the bonds directed above the plane of the two rings alternate from axial to equatorial and back if we proceed around the rings from C-1 to C-10 in numerical order. The bonds directed below the rings also alternate in a complementary fashion.
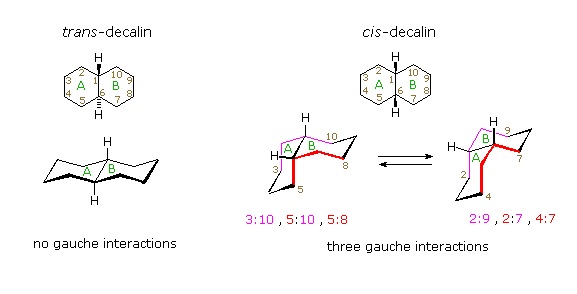
Conformational descriptions of cis- decalin are complicated by the fact that two energetically equivalent fusions of chair cyclohexanes are possible, and are in rapid equilibrium as the rings flip from one chair conformation to the other. In each of these all chair conformations the rings are fused by one axial and one equatorial bond, and the overall structure is bent at the ring fusion. In the conformer on the left, the red ring (B) is attached to the blue ring (A) by an axial bond to C-1 and an equatorial bond to C-6 (these terms refer to ring A substituents). In the conformer on the right, the carbon bond to C-1 is equatorial and the bond to C-6 is axial. Each of the angular hydrogens (Hae or Hea) is oriented axial to one of the rings and equatorial to the other. This relationship reverses when double ring flipping converts one cis-conformer into the other.
Cis-decalin is less stable than trans-decalin by about 2.7 kcal/mol (from heats of combustion and heats of isomerization data). This is due to steric crowding (hindrance) of the axial hydrogens in the concave region of both cis-conformers, as may be seen in the model display activated by the following button. This difference is roughly three times the energy of a gauche butane conformer relative to its anti conformer. Indeed three gauche butane interactions may be identified in each of the cis-decalin conformations, as will be displayed by clicking on the above conformational diagram. These gauche interactions are also shown in the model.
Steroids in which rings A and B are fused cis, such as the example on the right, do not have the same conformational mobility exhibited by cis-decalin. The fusion of ring C to ring B in a trans configuration prevents ring B from undergoing a conformational flip to another chair form. If this were to occur, ring C would have to be attached to ring B by two adjacent axial bonds directed 180º apart. This is too great a distance to be bridged by the four carbon atoms making up ring C. Consequently, the steroid molecule is locked in the all chair conformation shown here. Of course, all these steroids and decalins may have one or more six-membered rings in a boat conformation. However the high energy of boat conformers relative to chairs would make such structures minor components in the overall ensemble of conformations available to these molecules.
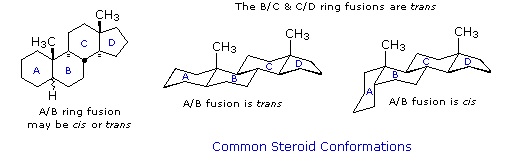
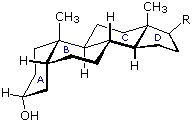
Much like cyclohexanes substituents (Section 4-7) on a steroid ring system can be either an axial or equatorial position. Fused rings makes steroids ridge and unable to undergo cyclohexane ring-flips Because of sterics, substituents in the equatorial posltion tend to be more energetically favorable. The -OH group present in cholesterol is in the more stable equatorial position while the two -CH3 groups on the steroid ring are both in an axial position.

Steroid Hormones
Hormones are chemical messengers that are released in one tissue and transported through the circulatory system to one or more other tissues. One group of hormones is known as steroid hormones because these hormones are synthesized from cholesterol, which is also a steroid. There are two main groups of steroid hormones: adrenocortical hormones and sex hormones.
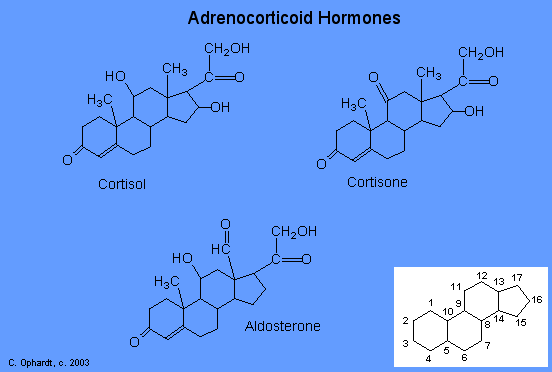
The adrenocortical hormones, such as aldosterone and cortisol (Table 27.6.1), are produced by the adrenal gland, which is located adjacent to each kidney. Aldosterone acts on most cells in the body, but it is particularly effective at enhancing the rate of reabsorption of sodium ions in the kidney tubules and increasing the secretion of potassium ions and/or hydrogen ions by the tubules. Because the concentration of sodium ions is the major factor influencing water retention in tissues, aldosterone promotes water retention and reduces urine output. Cortisol regulates several key metabolic reactions (for example, increasing glucose production and mobilizing fatty acids and amino acids). It also inhibits the inflammatory response of tissue to injury or stress. Cortisol and its analogs are therefore used pharmacologically as immunosuppressants after transplant operations and in the treatment of severe skin allergies and autoimmune diseases, such as rheumatoid arthritis.
Table 27.6.1 Representative Steroid Hormones and Their Physiological Effects
| Hormone |
Effect |
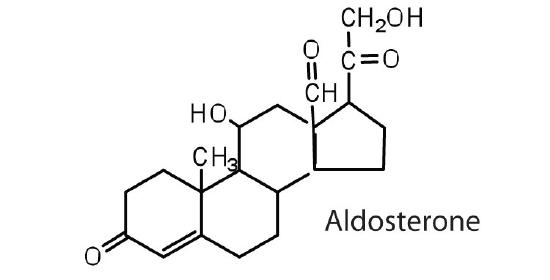 |
regulates salt metabolism; stimulates kidneys to retain sodium and excrete potassium |
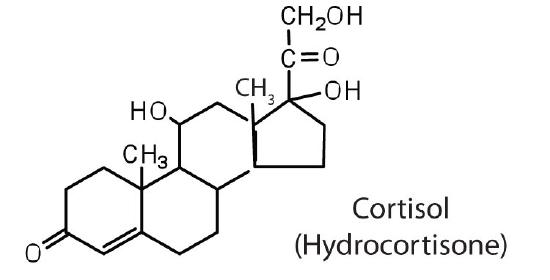 |
stimulates the conversion of proteins to carbohydrates |
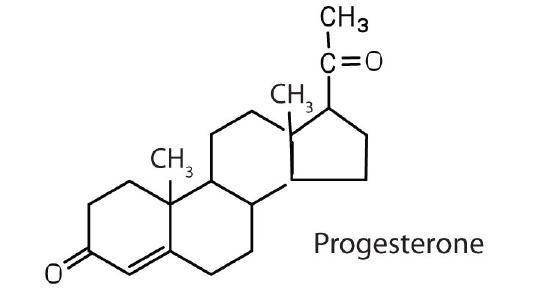 |
regulates the menstrual cycle; maintains pregnancy |
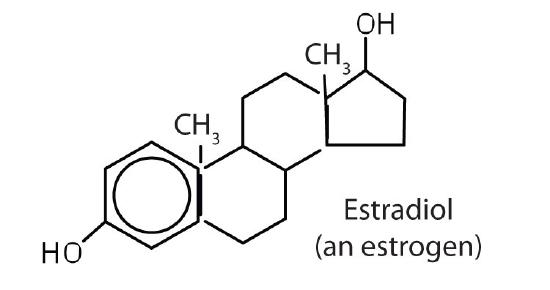 |
stimulates female sex characteristics; regulates changes during the menstrual cycle |
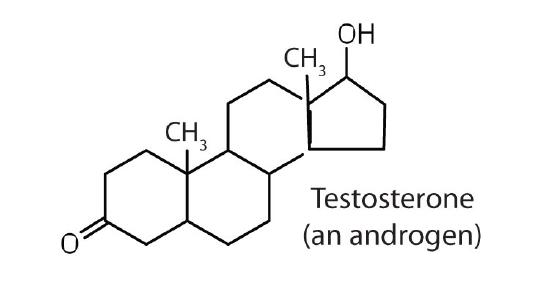 |
stimulates and maintains male sex characteristics |
The sex hormones are a class of steroid hormones secreted by the gonads (ovaries or testes), the placenta, and the adrenal glands. Testosterone and androstenedione are the primary male sex hormones, or androgens, controlling the primary sexual characteristics of males, or the development of the male genital organs and the continuous production of sperm. Androgens are also responsible for the development of secondary male characteristics, such as facial hair, deep voice, and muscle strength. Two kinds of sex hormones are of particular importance in females: progesterone, which prepares the uterus for pregnancy and prevents the further release of eggs from the ovaries during pregnancy, and the estrogens, which are mainly responsible for the development of female secondary sexual characteristics, such as breast development and increased deposition of fat tissue in the breasts, the buttocks, and the thighs. Both males and females produce androgens and estrogens, differing in the amounts of secreted hormones rather than in the presence or absence of one or the other.
Sex hormones, both natural and synthetic, are sometimes used therapeutically. For example, a woman who has had her ovaries removed may be given female hormones to compensate. Some of the earliest chemical compounds employed in cancer chemotherapy were sex hormones. For example, estrogens are one treatment option for prostate cancer because they block the release and activity of testosterone. Testosterone enhances prostate cancer growth. Sex hormones are also administered in preparation for sex-change operations, to promote the development of the proper secondary sexual characteristics. Oral contraceptives are synthetic derivatives of the female sex hormones; they work by preventing ovulation.
Adrenocorticoid Hormones
The adrenocorticoid hormones are products of the adrenal glands ("adrenal" means adjacent to the renal (kidney). The most important adrenocorticoid is aldosterone, which regulates the reabsorption of sodium and chloride ions in the kidney tubules and increases the loss of potassium ions. Aldosterone is secreted when blood sodium ion levels are too low to cause the kidney to retain sodium ions. If sodium levels are elevated, aldosterone is not secreted, so that some sodium will be lost in the urine. Aldosterone also controls swelling in the tissues.
Cortisol, the most important glucocortinoid, has the function of increasing glucose and glycogen concentrations in the body. These reactions are completed in the liver by taking fatty acids from lipid storage cells and amino acids from body proteins to make glucose and glycogen.
In addition, cortisol and its ketone derivative, cortisone, have the ability to inflammatory effects. Cortisone or similar synthetic derivatives such as prednisolone are used to treat inflammatory diseases, rheumatoid arthritis, and bronchial asthma. There are many side effects with the use of cortisone drugs, so there use must be monitored carefully.

Exercise \(\PageIndex{1}\)
The following molecule is cholic acid. Draw it showing the chair conformations. Determine if the three fused bonds have a cis or trans configuration. Determine if the OH groups are axial or equatorial.
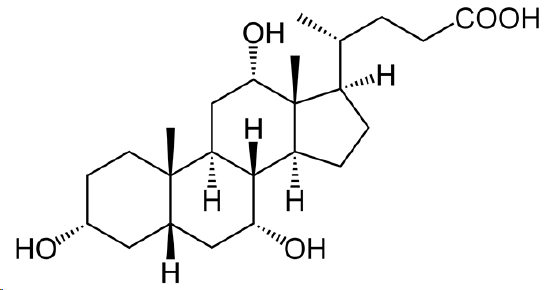
- Answer
-
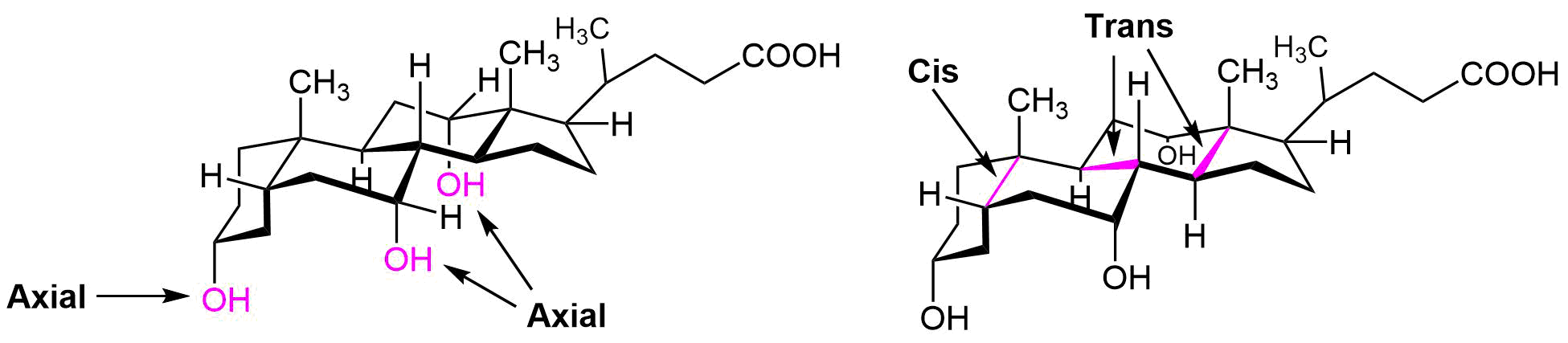
Exercise \(\PageIndex{2}\)
Draw the following decalin molecules in chair conformations. Determine if the -CH3 groups are axial or equatorial.
a)

b)

- Answer
-
a)

b)