17.9: Phenols and Their Uses
- Page ID
- 36353
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to describe two methods that can be used to obtain phenol on an industrial scale.
One substance that has become symbolic of the struggle between industrial development and environmental protection is dioxin. The name dioxin is used to refer to a family of compounds having a basic structure in which two benzene rings are joined by two oxygen atoms:

However, media references to dioxin are usually in connection with 2,3,7,8‑tetrachlorodibenzo‑ p‑dioxin, or TCDD:
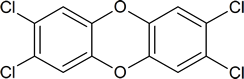
TCDD is a by‑product formed in the manufacture of trichlorophenol, an intermediate in the production of the herbicide 2,4,5‑T (one of the ingredients of the infamous Agent Orange), in the manufacture of hexachlorophene, by pulp and paper mills that use chlorine to produce white paper, and as a result of the incineration of municipal refuse.

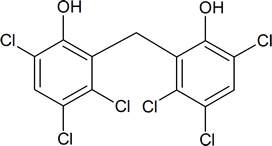
The equation given below shows how 2,3,7,8‑tetrachlorodibenzo‑ p‑dioxin is formed during the manufacture of 2,4,5‑trichlorophenol, an intermediate produced in the synthesis of 2,4,5‑T. The process involves the reaction of 1,2,4,5‑tetrachlorobenzene with base at a temperature of about 160°C.
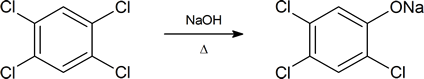

The possible hazards presented by dioxin first became apparent in 1957, when some German workers involved in the manufacture of 2,4,5‑T developed chloracne—a skin condition resembling acne. The precise toxicological effects of dioxin on humans is open to debate, but as little as 0.6 mg per kg of body weight will kill 50% of guinea pigs injected with TCDD within a specified time.
Dow’s Process
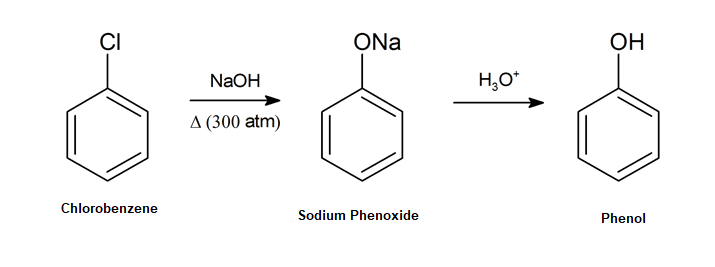
An early commercial preparation of phenol in the late 19th and early 20th century was by the hydrolysis of chlorobenzene with strong base to produce a sodium phenoxide intermediate, which affords phenol upon acidification.
Cumene Process
Developed in the 1940s and currently responsible for most industrial phenol production this process involves isopropylbenzene—commonly known as cumene. Treatment of cumene with oxygen in air generates 2-hydroperoxypropan-2-ylbenzene (cumene hydroperoxide) through a radical pathway. When hydrolyzed in acidic medium the peroxide intermediate produces phenol and acetone, which are both valuable chemical products.
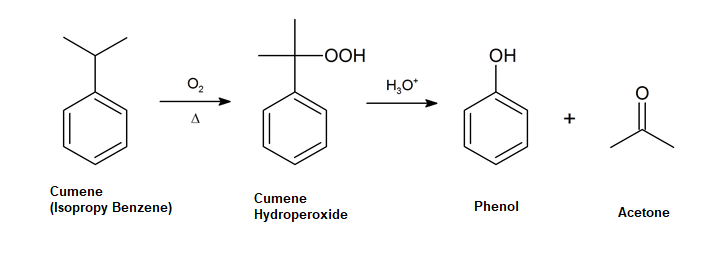
Mechanism
Protonation and water loss
Initially cumene react with oxygen (O2) to form cumene hydroperoxide. The -OH oxygen of the peroxide is protonated creating a good leaving group. This is following by a rearrangement where the phenyl group shifts form a carbon to an oxygen with the simultaneous loss of water.
Addition of water and proton transfer
The resulting carbocation intermediate is attacked by a nucleophilic water to form a protonated hemiacetal. A hemiacetal is a compound with an ether group (-OR) and a hydroxide group (-OH) bonded to the same carbon atom. A proton transfer occurs with the oxygen near the ring taking the extra hydrogen.
Elimination
An E2 reaction occurs which forms the C=O bond in the product acetone, and eliminating phenol as the other product.
To Your Health: Phenols and Us
Phenols are widely used as antiseptics (substances that kill microorganisms on living tissue) and as disinfectants (substances intended to kill microorganisms on inanimate objects such as furniture or floors). The first widely used antiseptic was phenol. Joseph Lister used it for antiseptic surgery in 1867. Phenol is toxic to humans, however, and can cause severe burns when applied to the skin. In the bloodstream, it is a systemic poison—that is, one that is carried to and affects all parts of the body. Its severe side effects led to searches for safer antiseptics, a number of which have been found.
An operation in 1753, painted by Gaspare Traversi, of a surgery before antiseptics were used.
One safer phenolic antiseptic is 4-hexylresorcinol (4-hexyl-1,3-dihydroxybenzene; resorcinol is the common name for 1,3-dihydroxybenzene, and 4-hexylresorcinol has a hexyl group on the fourth carbon atom of the resorcinol ring). It is much more powerful than phenol as a germicide and has fewer undesirable side effects. Indeed, it is safe enough to be used as the active ingredient in some mouthwashes and throat lozenges.
The compound 4-hexylresorcinol is mild enough to be used as the active ingredient in antiseptic preparations for use on the skin.
In addition to acting as an antiseptic, phenol is also a useful precursor in many chemical syntheses to produce pharmaceuticals, food preservatives, polymers, resins and adhesives. Phenolics are also present in a number of biological systems and natural products such as neurotransmitters, flavoring agents, and vitamins to name a few.
Bisphenol A
In the United States, single-serve bottled water is the fastest growing beverage of choice. However, drinking tap water creates less pollution and uses less energy and natural resources than transporting and manufacturing plastic water bottles. Unlike soda and other carbonated beverages, there is no deposit on water bottles and therefore fewer are recycled by consumers. Nationally, only 10% of plastic water bottles are recycled creating large quantities of waste. Many water bottles consist of a polycarbonate plastic made with Bisphenol A (BPA).
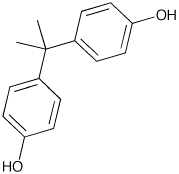
Figure 1: Bisphenol A
BPA was first synthesized by Thomas Zincke of the University of Marburg, Germany in 1905. Zincke did not propose uses for BPA however scientists discovered the many uses of BPA in 1953. Polycarbonate plastics became a commonly used commercial product in the 1950's.
BPA was created from a condensation reaction of phenol and acetone with hydrogen chloride, an acid catalyst, and a promoter such as methyl mercaptan. Once formed by this reaction, BPA is washed with water, neutralized with calcium hydroxide and distilled under vacuum. BPA can also be purified further by distillation and extractive crystallisation. Higher purity BPA is used to make polycarbonate plastics while the lower purity BPA is used to make Epoxy Resin. BPA's IUPAC name is 4,4'-dihydroxy-2,2,-diphenylpropane. It's chemical formula is C15H16O2. The production of BPA produces H2O (see image below) and therefore requires a condensation reaction for production.
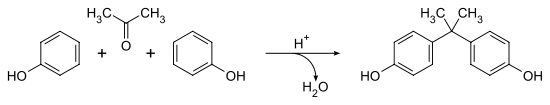
Figure 2 The production of BPA
A concern with the use of BPA in water bottles is the potential for leaching into the water which is then consumed. When BPA is present in the human body, it mimics the hormone estrogen and is capable of binding to estrogen receptors. In doing so, it changes the genes in the body that are expressed which triggers changes in hormone concentration, enzyme function and protein synthesis.
Using plastic water bottles that contain BPA is damaging both to the environment and human health. A greener alternative to the use of plastic water bottles is filtered tap water carried in refillable stainless steel containers.
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.
- Layne Morsch (University of Illinois Springfield)

