8.2: Halogenation of Alkenes - Addition of X₂
- Page ID
- 31466
After completing this section, you should be able to
- write the equation for the reaction of chlorine or bromine with a given alkene.
- identify the conditions under which an addition reaction occurs between an alkene and chlorine or bromine.
- draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine.
- write the mechanism for the addition reaction that occurs between an alkene and chlorine or bromine, and account for the stereochemistry of the product.
Make certain that you can define, and use in context, the key terms below.
- anti stereochemistry
- bromonium ion
In the laboratory you will test a number of compounds for the presence of a carbon-carbon double bond. A common test is the decolourization of a reddish-brown bromine solution by an alkene.
The two-step mechanism shown in the LibreText pages gives you an idea of how the reaction between an alkene and a halogen occurs. Note the formation of the bridged bromonium ion intermediate and the anti stereochemistry of the final product because the two bromine atoms come from opposite faces of the double bond.
Additional evidence in support of the bromonium ion mechanism comes from the results obtained when an alkene (such as cyclopentene) reacts with bromine in the presence of sodium chloride (see Figure 8.2: Reaction of an alkene with bromine in the presence of sodium chloride, below).
Once formed, the bromonium ion is susceptible to attack by two nucleophiles—chloride ion and bromide ion—and, in fact, a mixture of two products (both produced by anti attack) is formed.
Halogens can act as electrophiles to which can be attacked by a pi bond from an alkene. Pi bonds represents a region of electron density and therefore function as a nucleophiles. How is it possible for a halogen to obtain positive charge to be an electrophile?
Introduction
A halogen molecule, for example Br2, approaches a double bond of the alkene, electrons in the double bond repel electrons in the bromine molecule causing polarization of the halogen-halogen bond. This creates a dipole moment in the halogen-halogen bond. Heterolytic bond cleavage occurs and one of the halogens obtains a positive charge and reacts as an electrophile. The reaction of the addition is not regioselective but is stereoselective. Stereochemistry of this addition can be explained by the mechanism of the reaction. In the first step, the electrophilic halogen (with the positive charge) approaches the pi bond and 2p orbitals of the halogen bond with two carbon atoms creating a cyclic ion with a halogen as the intermediate. In the second step, the remaining halide ion (halogen with the negative charge) attacks either of the two carbons in the cyclic ion from the back side of the cycle as in the SN2 reaction. Therefore stereochemistry of the product is anti addition of vicinal dihalides.
\[\ce{R_2C=CR_2 + X_2 \rightarrow R_2CX-CR_2X} \tag{8.2.1} \]
Step 1: In the first step of the addition the Br-Br bond polarizes, heterolytic cleavage occurs and Br with the positive charge forms a cyclic intermediate with the two carbons from the alkene.
Step 2: In the second step, bromide anion attacks either carbon of the bridged bromonium ion from the back side of the ring. The bromine atom in the bromonium ion acts as a shield in a way, forcing the bromonium anion to attack from the opposite side as it. The result of this is the ring opening up with the two halogens on opposite sides as each other. This is anti stereochemistry, which is defined as the two bromine atoms come from opposite faces of the double bond. The product is that the bromines add on trans to each other.
Halogens that are commonly used in this type of the reaction are: \(Br\) and \(Cl\). In thermodynamical terms \(I\) is too slow for this reaction because of the size of its atom, and \(F\) is too vigorous and explosive.
Because the halide ion can attack any carbon from the opposite side of the ring it creates a mixture of steric products. Optically inactive starting material produce optically inactive achiral products (meso) or a racemic mixture.
Electrophilic addition mechanism consists of two steps.
Before constructing the mechanism let us summarize conditions for this reaction. We will use Br2 in our example for halogenation of ethylene.
| Nucleophile | Double bond in alkene |
| Electrophile | Br2, Cl2 |
| Regiochemistry | not relevant |
| Stereochemistry | ANTI |
Summary
Halogens can act as electrophiles due to polarizability of their covalent bond. Addition of halogens is stereospecific and produces vicinal dihalides with anti addition.
What is the mechanism of adding Cl2 to the cyclohexene?
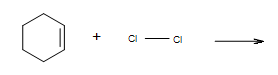
- Answer
-
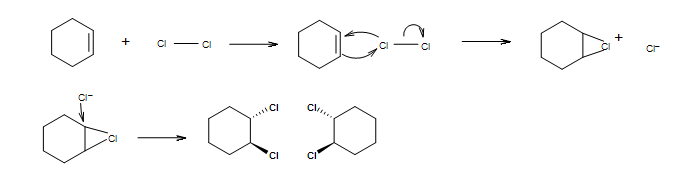
A reaction of Br2 molecule in an inert solvent with alkene follows?
- syn addition
- anti addition
- Morkovnikov rule
- Answer
-
b
- Answer
-
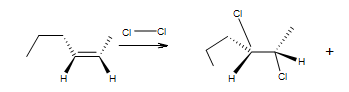 enantiomer
enantiomer
- Answer
-
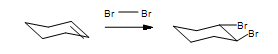
Predict the products for 1,2-dimethylcyclopentene reacting with Br2 with proper stereochemistry.
- Answer
-
Predict the products for 1,2-dimethylcyclpentene reacting with HCl, give the proper stereochemistry. What is the relationship between the two products?
- Answer
-
These compounds are enantiomers.
References
- Vollhard,K.Peter C., and Neil E.Schore.Organic Chemistry:Structure and Function. New York: W.H.Freeman and Company 2007
- Chemistry-A European Journal 9 (2003) :1036-1044

