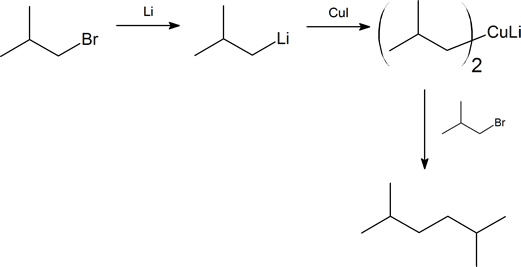10.7: Organometallic Coupling Reactions
- Page ID
- 31500
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write an equation for the formation of an alkyllithium from an alkyl halide.
- write an equation for the formation of a lithium dialkylcopper (Gilman) reagent from an alkyllithium and copper(I) iodide.
- write an equation for the coupling of a lithium dialkylcopper reagent with an alkyl halide (i.e., a Corey-House synthesis).
- draw the structure of the product formed from a given Corey-House synthesis.
- identify the reagents needed to convert two given organohalides to a specified hydrocarbon through a Corey-House synthesis.
Make certain that you can define, and use in context, the key terms below.
- Corey-House synthesis
- pheromone
A pheromone is a chemical released by members of one species to cause specific behavioural or physiological changes in other members of the same species. Examples include sex pheromones, alarm pheromones and trail pheromones.
The Corey-House synthesis provides us with a method of coupling together two alkyl groups through the formation of a new carbon-carbon bond. The product of such a reaction is an alkane, and this synthetic method gives us a route for the preparation of unsymmetrical alkanes. The method was developed during the late 1960s by E. J. Corey and Herbert House working independently at Harvard University and Massachusetts Institute of Technology, respectively. The overall synthetic route is shown on the next page. Note that R and R′ represent alkyl groups, which can be the same or different, and X represents a halogen (preferably bromine or iodine).
In order to obtain a good yield of alkane, both R′X and RX should be primary alkyl halides. However, the experimental procedure can be modified so that this synthesis can be carried out using a wide range of alkyl, aryl, vinyl, benzyl and allyl halides. A detailed discussion of these modifications is beyond the scope of this course, but you should be aware of possible limitations in the use of the Corey-House synthesis.
Note: In some textbooks, lithium diorganocoppers are referred to as lithium dialkylcoppers, or cuprates.
Gilman Reagents
Another important reaction exhibited by organometallic reagents is metal exchange. Organolithium reagents react with cuprous iodide to give a lithium diorganocopper reagent (also called diorganocuprates), which often is referred to as a Gilman reagent. Remember that organolithium reagents are formed by a reaction of lithium metal with an organohalide. Lithium diorganocopper reagents are considered a source of carbanion-like nucleophiles similar to Grignard and Organolithium reagents. However, the reactivity of lithium diorganocuprate reagents is slightly different and this difference will be exploited in specific situations. Diorganocuprate reagents are made from the reaction of two equivalents of an organolithium reagent and copper (I) iodide (CuI). The created lithium diorganocuprate reagent acts as a source of R:-
lithium diorganocopper (Gilman reagent)
General Reaction
Example
\(\ce{2 CH3Li + CuI -> (CH3)2CuLi + LiI}\)
Coupling Reactions with Gilman Reagents
Gilman reagents undergo a coupling reaction with organochlorides, bromides, and iodides to form a carbon-carbon bond. During the reaction one of the alkyl groups from the Gilman reagent replaces the halogen atom in the organohalide. This reaction is useful in organic synthesis because it allows a larger molecule to be built from smaller fragments. Most alkyl halides, including aryl and vinyl halides, are capable of undergoing this reaction.
General Reaction
Examples
Mechanism
The mechanism begins with copper in the Gilman reagent being oxidized from Cu+1 to Cu+3. Losing electrons allows the copper atom to act as a nucleophile and undergo an SN2 like substitution reaction with the alkyl halide. The resulting neutral tri-organocopper intermediate undergoes reductive elimination, reducing Cu+3 to Cu+1, and creating the alkyl coupling product.
The Suzuki-Miyaura Reaction
The reactions of diorganocopper reagents with organohalides are related to the processes that occur with other organometallic reagents such as organopalladiums. Suzuki-Miyaura coupling (or Suzuki coupling) is a metal-catalyzed reaction, typically with Pd, between an alkenyl (vinyl), aryl, or alkynyl organoborane (boronic acid or boronic ester, or special cases with aryl trifluoroborane) and halide or triflate under basic conditions. This reaction is used to create carbon-carbon bonds to produce conjugated systems of alkenes, styrenes, or biaryl compounds. Organopalladium reagents are often preferred over diorganocopper reagents, because only a catalytic amount of the organopalladium is necessary rather than a full equivalent which makes the reaction less toxic.
General Reaction
Examples
Mechanism
The general catalytic cycle for Suzuki cross coupling involves three fundamental steps: oxidative addition, transmetalation, and reductive elimination as demonstrated in the figure below.
The oxidative addition of aryl halides to the Pd(0) complex gives an intermediate 1, a Pd(II) species. Under the participation of base, an organoborane compound reacts with intermediate 1 in a transmetalation step to afford intermediate 2.
This is followed by reductive elimination to give the desired coupling product and regenerate the original Pd(0) species. Depending on different catalytic systems with various catalysts, ligands, and solvents, there are additional processes in the catalytic cycle, including ligand or solvent association and dissociation.
Starting with alkyl halides containing no more than four carbon atoms, how would you synthesize each of the following alkanes?
- 2,5-dimethylhexane
- 2-methylhexane
- Answer
- Notice that in (a), both the alkyl halides are primary. This fact should ensure a good yield of product.
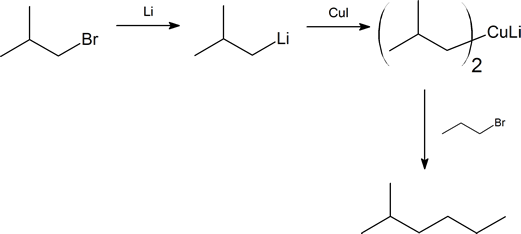
- In (b) we have the choice of using 2-bromopropane and 1-bromobutane, or 1-bromo-2-methylpropane and 1-bromopropane. We chose the latter as it enables us to use two primary alkyl halides, and hence a simpler procedure.