7.11: Infra-red Spectra as Evidence of Carboxylic Acid Derivative Structure
- Page ID
- 354438
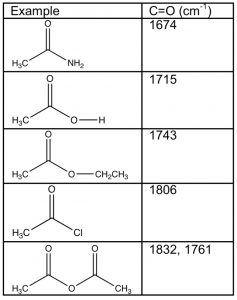
Carboxylic acids and their derivatives have a number of features in common, the most obvious being that the carbonyl carbon has three bonds to electronegative elements—the carbonyl carbon is, therefore, in a higher oxidation state than aldehydes and ketones. However, the nature of the electronegative atom bonded to the carbonyl does impact the properties of the molecule as a whole. For example, if we examine the carbonyl absorptions of these derivatives, we find quite a wide range of frequencies. Recall that most aldehydes and ketones absorb around \(1710-30 \mathrm{cm}^{-1}\), similar to the value displayed by carboxylic acids. Derivatives of carboxylic acids, however, range from around \(1670 \mathrm{cm}^{-1}\) for amides to 1810-30 cm–1 for acid chlorides and anhydrides. How can we explain the difference and use it to predict (and explain) differences in the properties of these derivatives?
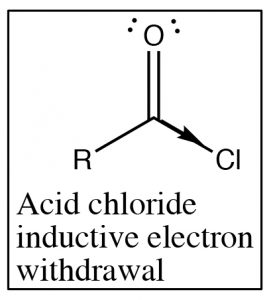
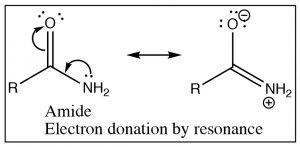
Recall that the IR absorption frequency of carbonyls depends on the energy required to stretch the bond (which is determined by the bond energy). Since the amide absorption frequency is much lower than the absorption frequency of the acid chloride we can conclude that the carbonyl group in the amide requires less energy to stretch than the acid chloride (the bond is weaker). If we look at the structures of these two functional groups we see that both involve an electronegative element bonded to the carbonyl carbon, leading to electron withdrawal by induction through the sigma bond to the carbon. The difference between the two (amide and acid chloride) arises because the amide nitrogen can also donate its electron pair to the carbonyl oxygen. The result is that in amides there is significant overlap between the lone pair of the amide nitrogen and the carbonyl pi bond system. The \(\mathrm{C-O}\) bond now has less double bond character and therefore it takes less energy to stretch. In contrast chlorine (or any halogen) is not basic and so does not participate in this kind of resonance through the pi system. In acid chlorides, the chlorine is removing electron density from the carbon by induction through the pi system; there is more \(\mathrm{C=O}\) double bond character than in an amide and it takes more energy to stretch the carbonyl bond (leading to a higher IR absorption).
Electron donation from the nitrogen means that the lone pair on the amide nitrogen is not as available for donation to acids. This means that amides are not basic, or rather, nowhere near as basic as amines in which the lone pair is freely available for donation to an acid. This has important ramifications in biological systems. In polypeptides, which are amino acids linked by amide functional groups (circled below), amines are protonated at physiological \(\mathrm{pH}\) (they exist as \(\mathrm{RNH}_{3}{}^{+}\))[8]. It is an interesting thought experiment to predict how proteins and peptides would behave if amide nitrogens were more basic.
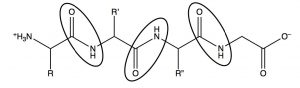
While there are relatively few naturally occurring acidic or basic side chains in polypeptides (from amino acids such as glutamic acid or lysine), every peptide is linked by innumerable amide bonds between the individual amino acids. If all these amide nitrogens were protonated, the peptides and proteins would take up very different structures (since they would have a large positive charge which would, in the absence of counter ions, repel other parts of the molecule).


