4.4: Eliminations
- Page ID
- 354357
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skeletal rearrangements are a drawback of exposing substrates to conditions in which the leaving group ionizes. Unfortunately, they are not the only complication—there is also the possibility that another type of reaction may occur—an elimination to produce an alkene.

In this case, the reaction proceeds through the same carbocation intermediate, and then a proton is eliminated from a carbon next to the carbocation (a \(\beta\) carbon).

An E1 elimination reaction
This is called an elimination reaction, and it is first-order since the rate-determining step is the formation of the carbocation, and so it is an \(\mathrm{E} 1\) reaction. In fact, \(\mathrm{S}_{\mathrm{N} 1\) reactions are often accompanied by \(\mathrm{E} 1\) reactions (and vice versa). If there is a possibility of forming more than one alkene (because there are different \(\beta\) carbons), usually the most substituted alkene is the major product.[4] For example, alcohols undergo \(\mathrm{E} 1\) eliminations when treated with concentrated sulfuric acid. In this case, there is no substitution product because the sulfate anion is not a good nucleophile (it is highly stabilized by resonance).

Here, the major product has three alkyl groups on the double bond, while the minor product only has two. In fact, this acid catalyzed dehydration of alcohols is quite a synthetically useful reaction, but if the substrate has the potential for rearrangements (i.e. the resulting carbocation can be stabilized by a hydride or alkyl shift), then there is the potential for the formation of even more products. For example:
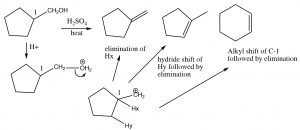
Elimination and Rearrangements
Obviously, these kind of rearrangements and eliminations are not synthetically useful on substrates that are prone to skeletal rearrangements. However, there is an elimination reaction that typically provides us with much more control.
The \(\mathrm{E} 2\) Reaction.
As we will see shortly, the synthesis of alkenes by elimination of \(\mathrm{H–L}\) (where L is a leaving group) is an important reaction, but we are much more likely to have control over the products if the reaction does not go through a carbocation. That is, if we can simultaneously eliminate both the \(\mathrm{H}^{+}\) and the leaving group there is less chance of side reactions. This reaction is an \(\mathrm{E} 2\) reaction (elimination second order), and is promoted by the presence of a strong base. For example, the reaction of t-butyl bromide with hydroxide (or any strong base), shown above. In this case, there is little substitution product, and instead the base simultaneously removes a proton from the \(\beta\) carbon as shown.


An E2 Elimination
The rate therefore depends on both the substrate and the base:\(\text { Rate }=k[\mathrm{RL}][\text{base}]\)—that is, a second order reaction. But wait—didn’t we see that strong bases are good nucleophiles? From the beginning, we have shown that methyl and primary substrates with strong nucleophiles undergo \(\mathrm{S}_{\mathrm{N} 2\) reactions. How can we bring about an elimination in this case? Well, just as a sterically hindered substrate will not undergo an \(\mathrm{S}_{\mathrm{N} 2\) reaction, we can use a sterically hindered base to avoid such reactions. If the base is too bulky around its reactive site, then it cannot approach the substrate at the electrophilic center, and will instead pick of a proton from one of the \(\beta\) carbons. One such base is the salt of t-butanol, potassium t-butoxide (\(\mathrm{tBuOK}\)), which is used to bring about \(\mathrm{E} 2\) eliminations for primary and secondary substrates.

Just as with \(\mathrm{E} 1\) reactions, the most substituted double bond is the major product:

Another factor that must be accounted for in \(\mathrm{E} 2\) reactions is that for such a reaction to occur, the leaving group and the proton that is eliminated must be in an orientation that allows the rehybridizing orbitals to overlap in the transition state.

This orientation is called antiperiplanar, and this need for a specific arrangement for the reaction to occur is called the stereoelectronic requirement.

Antiperiplanar (or trans diaxial) stereoelectronic requirement for \(\mathrm{E} 2\) eliminations
In systems where free rotation is possible, this lining up of the groups is not usually a problem, but if the elimination is to take place in a ring system, then the \(H\) and the leaving group must be trans and diaxial, otherwise the stereoelectronic requirement cannot be met.
So, for example, 1-bromo-2-methylcylohexane produces 3-methylcyclohexene, not 1- methylcyclohexene. This is because the hydrogen on the same carbon as the methyl group must be equatorial (if the methyl group is trans). Therefore the axial hydrogen on the other beta carbon is eliminated instead.
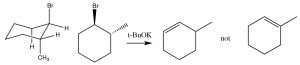
\(\mathrm{E} 2\) elimination requires a trans diaxial (antiperiplanar) conformation
| Nucleophile/base strength | Methyl | Primary | Secondary | Tertiary |
| Strong/strong e.g. \({}^{-} \mathrm{OCH}_{3}\) | \(\mathrm{S}_{\mathrm{N}} 2\) | \(\mathrm{S}_{\mathrm{N}} 2\) | \(\mathrm{E} 2\) | \(\mathrm{E} 2\) |
| Strong/weak e.g. \(\mathrm{RSH}\), halide ions | \(\mathrm{S}_{\mathrm{N}} 2\) | \(\mathrm{S}_{\mathrm{N}} 2\) | \(\mathrm{S}_{\mathrm{N}} 2\) | \(\mathrm{NR}\) |
| Weak/strong e.g. \({}^{-} \mathrm{OBu}^{\mathrm{t}}\), NaH | \(\mathrm{NR}\) | \(\mathrm{E} 2\) | \(\mathrm{E} 2\) | \(\mathrm{E} 2\) |
| Weak/weak e.g. \(\mathrm{H}_{2} \mathrm{O}\), \(\mathrm{CH}_{3} \mathrm{OH}\) | \(\mathrm{NR}\) | \(\mathrm{NR}\) | \(\mathrm{S}_{\mathrm{N}} 1 / \mathrm{E} 1\) | \(\mathrm{S}_{\mathrm{N}} 1 / \mathrm{E} 1\) |
One thing is certain: the interplay between substrate and solvent can be very confusing. It is impossible to memorize all the possible outcomes from a given set of reaction conditions, and although some generalizations can be made, the best way to manage all of this is to try to work through the reaction by writing a plausible mechanism. That being said, the following table summarizes some of the potential outcomes by type of substrate and strength of the nucleophile/base.


