4.7: Nitrogen Inversion Frequencies of Cyclic Imines
- Page ID
- 366661
Another interesting process which can be studied by NMR spectroscopy is the rate of inversion of nonplanar nitrogen carrying substituent groups. Ammonia has long been known from its rf spectrum to undergo inversion extremely rapidly. The corresponding inversion frequencies of various alkyl- and aryl-substituted amines have not been determined, but a variety of experiments have failed to provide optically active isomers of trisubstituted amines that are asymmetric solely because of the configuration at the nitrogen atom, presumably because inversion is too rapid. In 1940, Kincaid and Henriques9 published calculations which indicated that the rates of inversion of substituted nitrogen compounds would be much smaller if two of the substituents on nitrogen were connected together so as to form a three-membered N-C-C ring. However, prior and subsequent attempts to resolve such substituted ethylenimine derivatives into optically active forms were unsuccessful.
Nonetheless, the predictions of Kincaid and Henriques have been confirmed through the NMR spectra of cyclic imines.10
The proton resonance spectrum of N-ethylethylenimine at various temperatures is shown in Fig. 4-12. At room temperature, the typical three-four line pattern of the N-ethyl group is easily recognized and there remain two groups of lines to be assigned which arise from the ring protons. Models of N-ethylethylenimine show that, if the nitrogen is not planar, one would expect two kinds of ring protons-ones which are cis to the ethyl group and ones which are trans to the ethyl group.
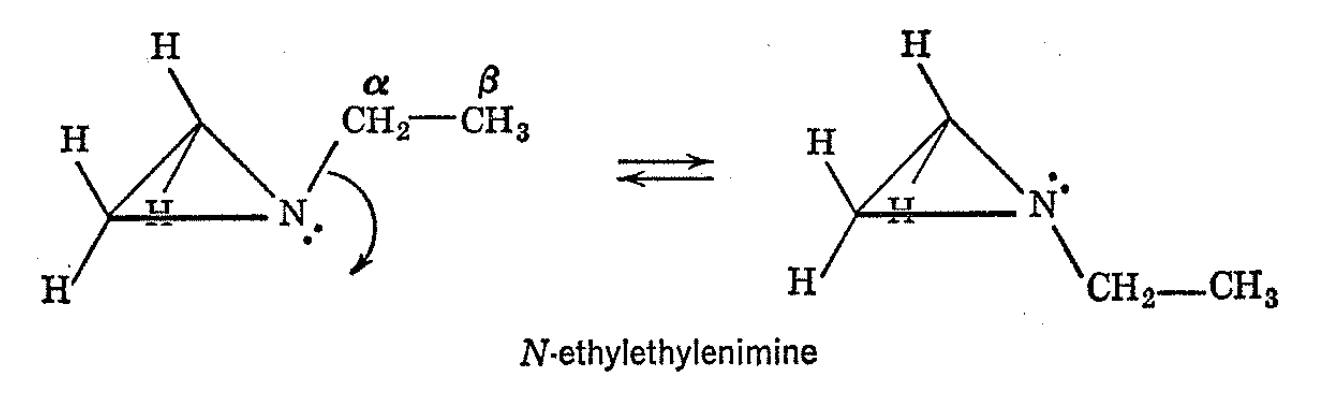
These will be in different magnetic environments and can account for the two observed peaks about 30 cps apart. Since nitrogen inversion results in exchange of the environments of the ring protons, the mean lifetime of an ethylenimine molecule before inversion occurs must be much longer than 0.015 sec if the cis- and trans-ring protons are to give separate sharp resonances. At higher temperatures, the inversion rate increases, and, finally, at 120°, the two ring-hydrogen resonances coalesce to a single line. The intermediate temperature is about 110°, and at this point the mean lifetime of the molecule before inversion is about 0.015 sec.
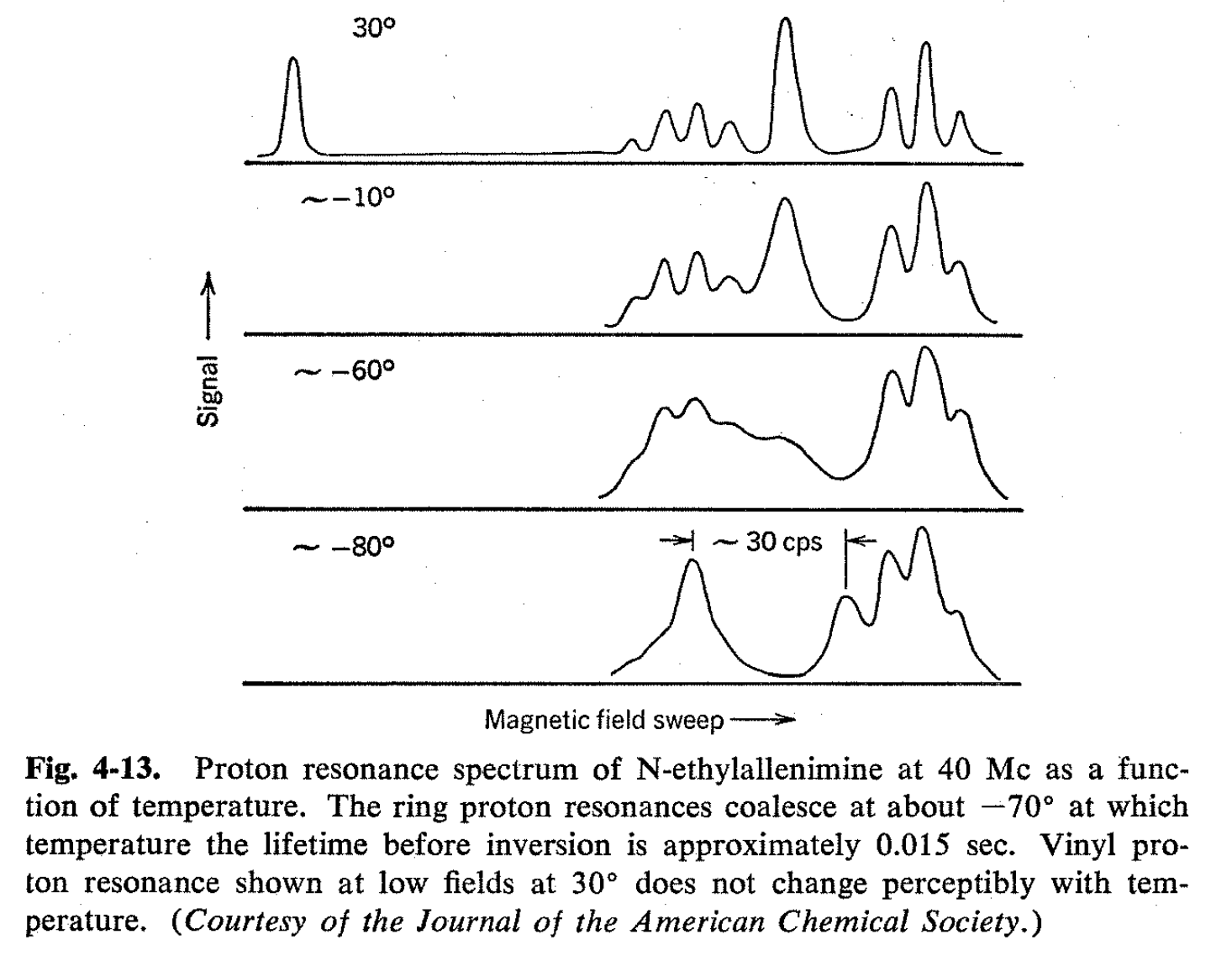
Effects of substituents on the inversion rates of cyclic imines have been studied by the NMR method, and it has been found that bulky groups either on the ring or on the nitrogen tend to increase the inversion rates, presumably by destabilizing the separate nonplanar configurations relative to the transition state. Unsaturated groups connected to either the ring or the nitrogen of ethylenimine cause the inversion rates to increase markedly. Presumably, with such substituents, the planar transition state is stabilized relative to the nonplanar ground state by resonance interaction between the unshared pair of electrons on nitrogen and the unsaturated centers. N-Ethylallenimine provides an excellent example of this type of behavior and the temperature dependence of its NMR spectrum is shown in Fig. 4-13.
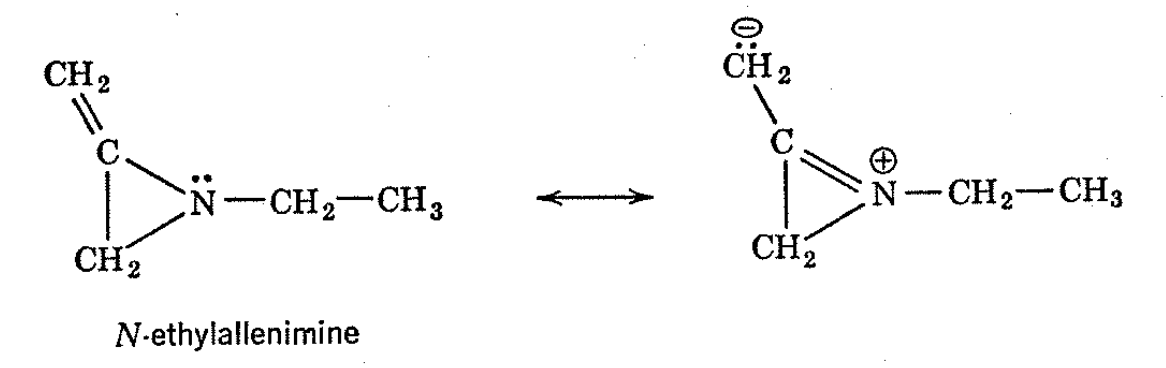
Solvents like water or alcohol which can form hydrogen bonds to the unshared electron pairs of ethylenimines substantially reduce the imine inversion frequencies. Presumably, the hydrogen bonds stabilize the nonplanar configuration by tending to anchor the unshared electron pairs on nitrogen.
9 J. F. Kincaid and F. C. Henriques, Jr., J. Am. Chem. Snc., 62, 1474 (1940).
10 A. T. Bottini and J. D. Roberts, J. Am. Chem. Soc., 78, 5126 (1956), and 80, 5203 (1958).


