4.6: Restricted Rotation in Ethane Derivatives
- Page ID
- 366660
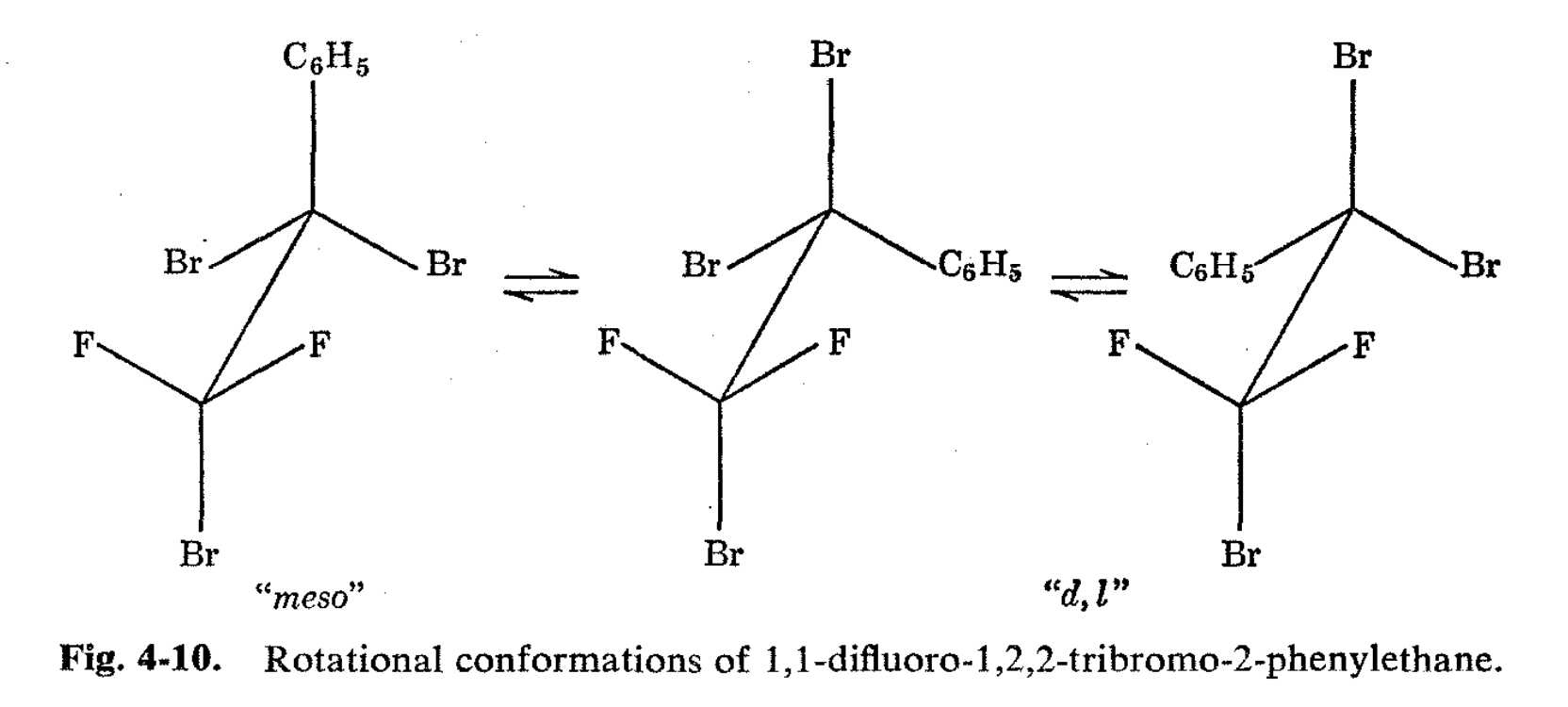
In favorable circumstances, the rates of rotation around C-C bonds in ethane derivatives can be shown by NMR methods to be slow.4,8 An excellent example is afforded by the behavior of 1,1-difluoro-1,2-dibromo-2,2-dichloroethane.8 The 19F nuclear resonance spectrum of this substance at room temperature shows a single sharp line. Inspection of the possible rotational conformations (Fig. 4-8) shows that a single 19F line is to be expected either as a result of averaging of the fluorines by rapid rotation or else, if rotation is slow, because the substance is "locked into" the "meso" conformation (I) with the 1,2-bromines trans to one another and the fluorines equivalently located. The other possible staggered conformations are seen to be a "d,l" pair (II and III) and to have nonequivalent fluorines. Rapid equilibrium of these two rotational isomers would average the fluorine spectrum to a single line because, as one isomer goes to the other, the fluorines exchange magnetic environments. Therefore, the rotational isomers must be rapidly equilibrated or else the substance must exist predominantly in a symmetrical form in order that only a single resonance line be obtained.
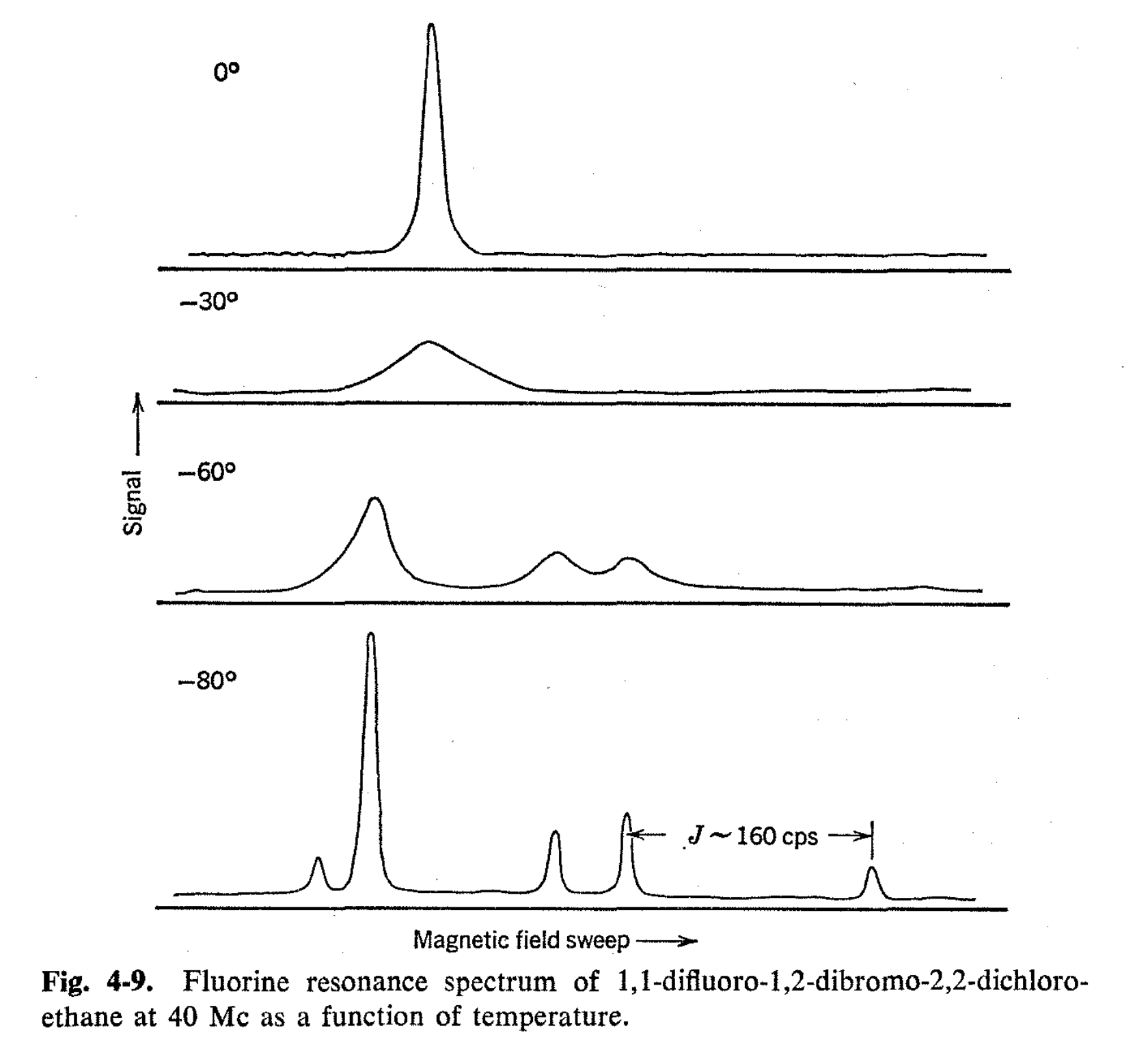
The 19F spectrum of 1,1-difluoro-1,2-dibromo-2,2-dichloroethane is quite temperature dependent, as is shown in Fig. 4-9. At -30°, the single line is seen to broaden substantially; at -60°, new lines commence to appear; and at -80°, five sharp lines are observed. These lines can be easily identified by their spin-spin coupling patterns. Four of the lines represent the spectra of the d,l pair II and III, which have nonequivalent fluorines, so that the fluorine resonances are shifted from one another and the concomitant spin-spin interactions give a quartet of lines with the outer lines being weakened by virtue of J being comparable with \(\delta\)H.
Since the rotational isomers II and III are mirror images, they will give identical spectra, and the symmetrical four-line pattern in Fig. 4-9 represents the contribution of this pair. The large single sharp resonance is due to the symmetrical "meso" rotational isomer with equivalent fluorines. At -80°, the rate of interconversion of these isomers must be much less than 200 times per second. On a purely statistical basis, the ratio of I to the isomers II and III should be 1:2. However, a rough analysis of the mixture at -80° by measuring the ratios of the signal intensities indicates that the composition is actually close to 1.4:1. This ratio is quite reasonable, since the conformation with two bromines trans to each other is expected to be sterically favored.
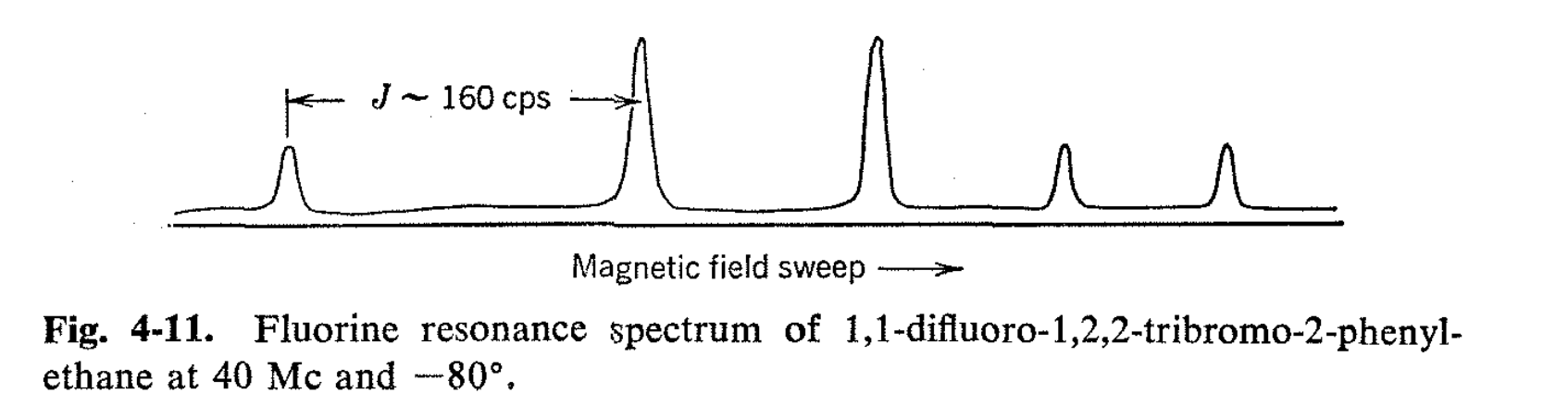
It is possible that valuable information as to relative steric sizes of groups will be obtainable through NMR studies of rotational isomer populations. An excellent example is afforded by 1,1-difluoro-1,2,2- tribromo-2-phenylethane. The 19F spectrum of this substance at room temperature shows a single line, while at -80° there are five lines as expected from a "freezing out" of the rotational isomers. The "meso" isomer of 1,1 -difluoro-1,2,2-tribromo-2-phenylethane has phenyl trans to bromine, and this would be expected to be the favorable conformation if phenyl were sterically larger than bromine as far as influencing the populations of the various configurations goes (see Fig. 4-10). However, the NMR spectrum (Fig. 4-11) shows that the meso isomer is actually present in far smaller concentration than the d,l pair, and in this case, we conclude that bromine acts as though it were larger than phenyl. Extensions of this type of experiment can clearly provide much information with regard to rotation about single bonds which is not easily available in any other way.
8 P. M. Nair and J. D. Roberts, J. Am. Chem. Soc., 79, 4565 (1957).


