4.4: Ethyl Acetoacetate and Its Enol Form
- Page ID
- 366658
Ethyl acetoacetate provides an interesting example of the use of NMR spectra for both a structural and kinetic analysis. The spectrum of the pure ester is shown in Fig. 4-6. The ethoxy group of the ester is easily identified by the typical four-three pattern of resonance lines. The other prominent resonances are due to the \(\alpha\) and \(\gamma\) protons. These are approximately in the theoretical ratio 2:3 and appear in the anticipated order with respect to field strength. Thus, the protons on the \(\alpha\)-carbon atom are adjacent to two electron-attracting carbonyl groups and give a resonance line at a considerably lower field than the protons on the \(\gamma\)-carbon atom, which are adjacent to only one carbonyl group. At room temperature, ethyl acetoacetate contains about 10 per cent of the corresponding enol form. The presence of this material is shown by the NMR spectrum, there being a small band in the vinyl hydrogen region and a hydrogen-bonded hydroxyl proton resonance at very low fields. The strength of these bands agrees with the composition as established by the Kurt Meyer titration. Apparently, the \(\gamma\)-methyl and ethoxy resonances of the enol form are not separated enough from those of the keto form to make them easily distinguishable in the appropriate regions.
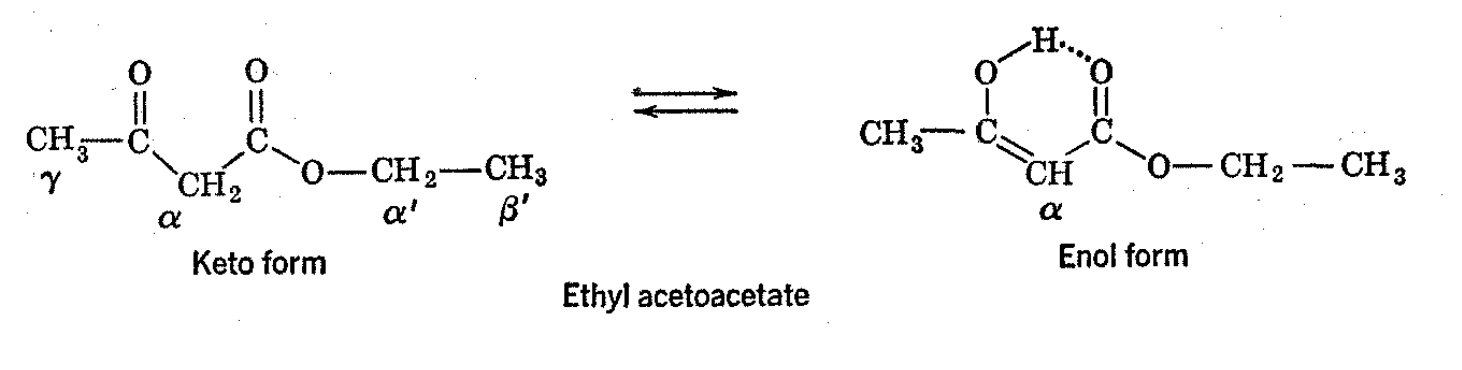
The detection of the separate resonances of the keto and enol forms shows that the enol and keto forms are not interconverted rapidly at room temperature, and this is in agreement with the observation that the enol and keto forms can be separated by "aseptic distillation" and separately preserved at low temperatures. The NMR spectrum of the equilibrium mixture of the ethyl acetoacetate tautomers at room temperature is markedly altered by the addition of a small amount of the sodium enolate (from dissolution of a small piece of sodium in the liquid), as shown in Fig. 4-6. The \(\alpha\)-proton resonance of the keto form and vinyl and 0-H resonances of the enol form disappear, and a new, rather broad band appears underneath the resonances of the \(\alpha\) hydrogens of the ethyl group. In the particular circumstances, exchange is occurring at an intermediate rate among the \(\alpha\)-keto, vinyl- , and hydroxylenol hydrogens. Cooling the mixture slows down the rate of exchange and the a-hydrogen line reemerges, although somewhat broadened. On heating, the exchange rate is increased and a new rather sharp average line of the exchanging protons is produced.
Separate experiments have shown that the chemical shifts of the resonance lines of the keto form of ethyl acetoacetate are not substantially altered by raising the temperature. With this information, it is possible to calculate that about 10 per cent of the enol is present in the rapidly exchanging mixture at 110 degrees, by virtue of the relative position of the average line with respect to the a-proton line of the keto form. The average consists of a small contribution of enol hydroxyl with a resonance line at a very low field, an equal-sized vinyl resonance at a much higher field, and a large contribution of \(\alpha\)-keto hydrogens at a still higher field. Thus, the equilibrium composition of the tautomers of ethyl acetoacetate probably does not change markedly with temperature. A comparable analysis with acetylacetone indicates that with this liquid there is a greater temperature dependence for the position of its keto-enol equilibrium.


