2.8: Chemical Shifts and Organic Structure Determinations. General Considerations
- Page ID
- 394068
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)a. Feist's Acid
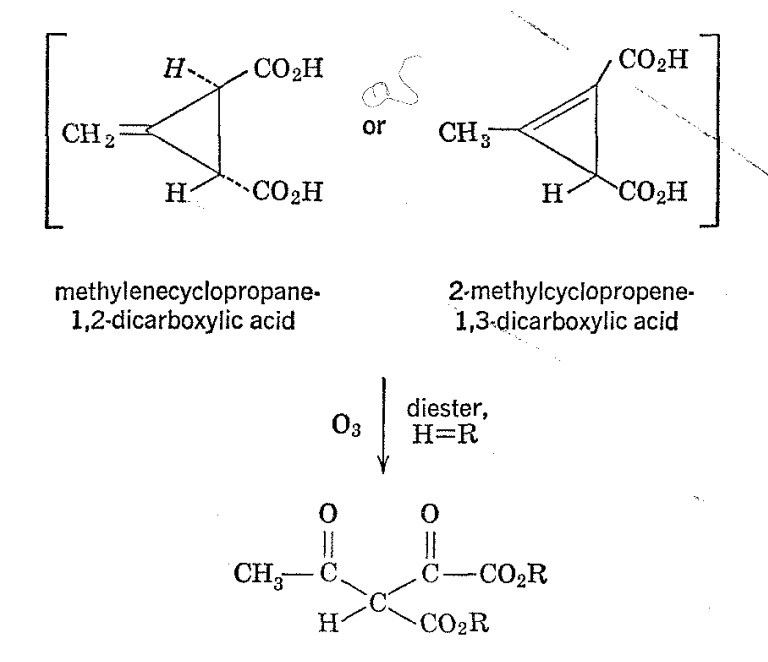
Measurements of chemical shifts and signal areas can be extremely valuable in determinations of structures of both simple and complicated molecules. An example is Feist's acid, originally postulated by Thorpe and coworkers,13 to be a methylcyclopropenedicarboxylic acid on the basis of chemical evidence-particularly, ozonization of the diethyl ester, which gave no formaldehyde but only a substance having properties appropriate to diethyl oxaloacetoacetate. Later, Ettlinger14 showed that a more likely structure for Feist's acid was trans-3-methylenecyclopropane-1,2-dicarboxylic acid.
Feist's acid is a high-melting solid, and satisfactory high-resolution spectra are usually obtainable only with nonviscous liquids or solutions. This is because in solid or viscous materials, the intermolecular magnetic effects of the various magnetic nuclei on one another are substantial and produce local variations in the total magnetic field. These result in a spread of precession frequencies even for nuclei of the same chemical kind, and such a spread in precession frequencies causes line broadening. In a nonviscous liquid, the molecules tumble over and over at a rapid rate and the eflect of magnetic nuclei in one molecule on the precession frequencies of nuclei in another molecule is averaged to zero.
The choice of a suitable solvent for determination of the NMR spectra of Feist's acid or similar substances illustrates several points of practical importance. Ideally, the solvent would have no resonance absorption of its own or else only a single line such as might serve as a calibration point. Carbon tetrachloride and carbon disulfide are common nonhydrogenous organic solvents in which, unfortunately, most polar organic compounds have only a limited solubility. A number of substances are available with only a single proton absorption, such as chloroform, acetone, benzene, cyclohexane, dimethyl sulfoxide, dioxane, and water, which nicely cover the range between polar and nonpolar solvents. However, even with these solvents, if the sample is only partly soluble, the increase in receiver sensitivity necessary to obtain a measurable signal may well produce a troublesome, large, and broad solvent peak. This can be avoided by using suitably deuterated solvents, and many of these, such as deuterochloroform, benzene, acetic acid, acetone, and, of course, heavy water, are commercially available. In general, concentrated solutions are desirable to give a good signal-to-noise ratio and to diminish the importance of signals due to solvent impurities.
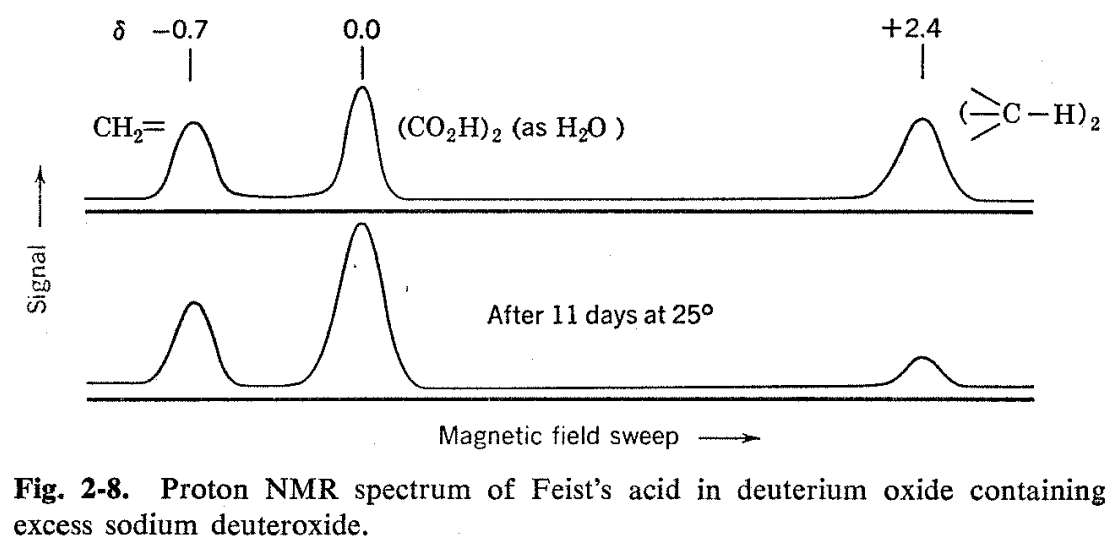
The proton resonance spectrum of Feist's acid was found to be most conveniently measured with the substance in the form of the sodium salt as obtained by dissolution in sodium deuteroxide-deuterium oxide solution.15 Use of D2O avoided a strong water peak and, in addition, gave a convenient calibration peak for the spectrum, because a twoproton water (or HDO) peak results from neutralization of the two carboxyl groups of the acid. As will be seen from Fig. 2-8, two other hydrogen resonances are observed. One of these appears in the vinyl hydrogen region as evinced by its \(\delta\) value of -0.7 X 106, while the other comes at a much higher field strength (\(\delta\) = +2.4 X l06). The areas of all three peaks are approximately the same, indicating that eack corresponds to two protons. The NMR spectrum of Feist's acid is decisive with respect to a choice between the Thorpe and Ettlinger structures. The Ettlinger structure is in complete agreement with the observed spectrum, while the Thorpe structure would require aliphatic methyl and alicyclic ring hydrogen resonances at high fields in the ratio of 3: 1 and no vinyl hydrogen resonance.
The study of Feist's acid gave a bonus observation in that the water peaks of the sodium deuteroxide solution (which contained some excess sodium deuteroxide) increased on standing at room temperature while the alicyclic hydrogen peaks decreased (see Fig. 2-8). This shows that the aliphatic hydrogens but not vinyl hydrogens exchanged slowly with the sodium deuteroxide-deuterium oxide solution at room temperature and, furthermore, proves that Feist's acid is not in rapid equilibrium under these conditions with molecules having the methylcyclopropenedicarboxylic acid structure because, in that case, the vinyl hydrogens would exchange as well.
Ettlinger16 has used the same technique to show that exchange of the vinyl hydrogens does occur at higher temperatures, and this is the first evidence that molecules with the Thorpe structure can be formed at all.
b. Ring-opening Reaction of 2-Chloro-3-phenylcyclobutenone
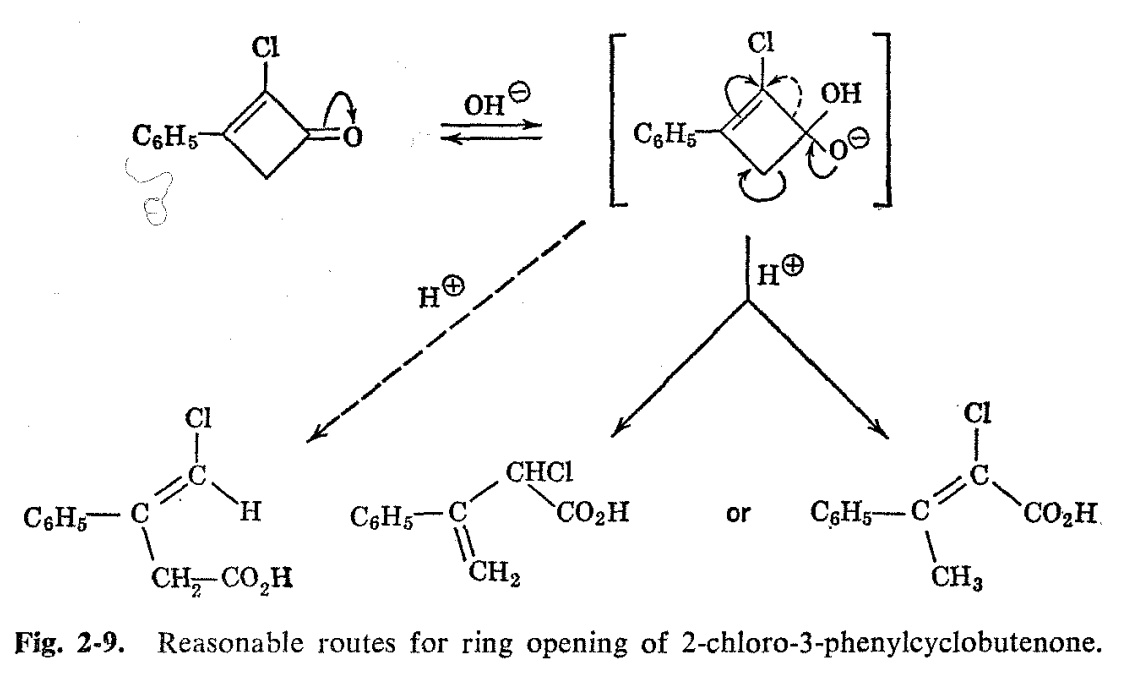
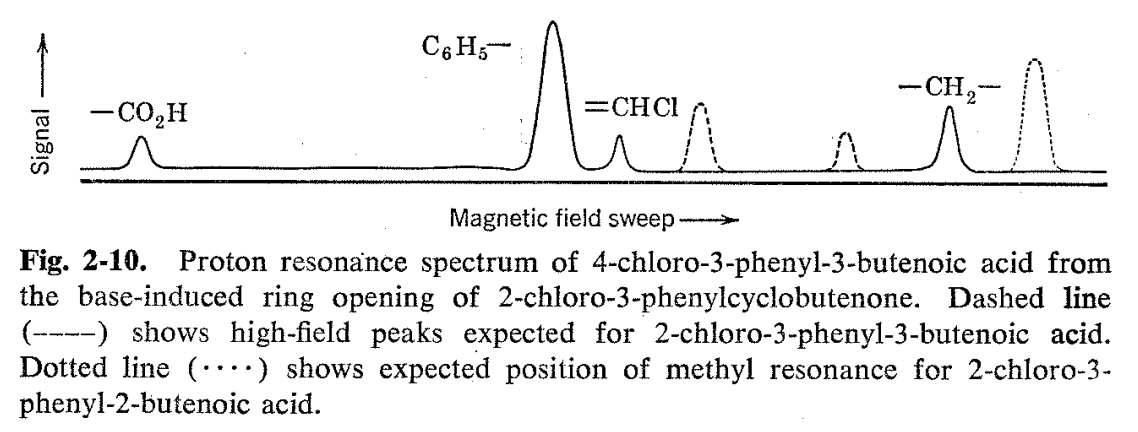
Another structure proof utilizing the same principles involves the phenylchlorobutenoic acid formed from ring opening of 2-chloro-3- phenylcyclobutenone with sodium hydroxide.17 Reasonable mechanisms leading to three isomeric acids can be written for this reaction, and a priori one might expect that formation of the conjugated isomer 2- chloro-3-phenylbutenoic acid would be favored (see Fig. 2-9). The NMR spectrum (Fig. 2-10) of the reaction product indicates otherwise. . Besides the carboxyl and phenyl hydrogen resonances which would be common to all three of the reasonably expected structures, there are two other peaks in the ratio of 1:2. The one-hydrogen resonance occurs in the vinyl hydrogen region, while the two-hydrogen resonance is at somewhat lower fields than a normal aliphatic hydrogen. We thus conclude that the product is 4-chloro-3-phenyl-3-butenoic acid formed by breaking the cyclobutenone ring between the 1- and 2-positions. The a priori expected structure is ruled out, since it would show no vinyl hydrogens and a simple three-hydrogen methyl resonance in the aliphatic hydrogen region. The line positions alone do not serve to rule out 2-chloro-3-phenyl-3-butenoic acid as a possibility, since it has both the vinyl- and aliphatic-type hydrogens, but the ratios of high-to-low field resonance lines for this compound would be reversed from what is actually observed.
c. Complex Organic Structures
The use of nuclear magnetic resonance can be profitably extended to even quite complex naturally occurring substances. An example taken from steroid chemistry is the tetracyclic ketol obtained by Johnson and coworkers18 by way of an aldol condensation and assigned the structure shown below by virtue of its conversion through the action of strong base and then by a long sequence of synthetic steps to naturally occurring steroidal hormones.
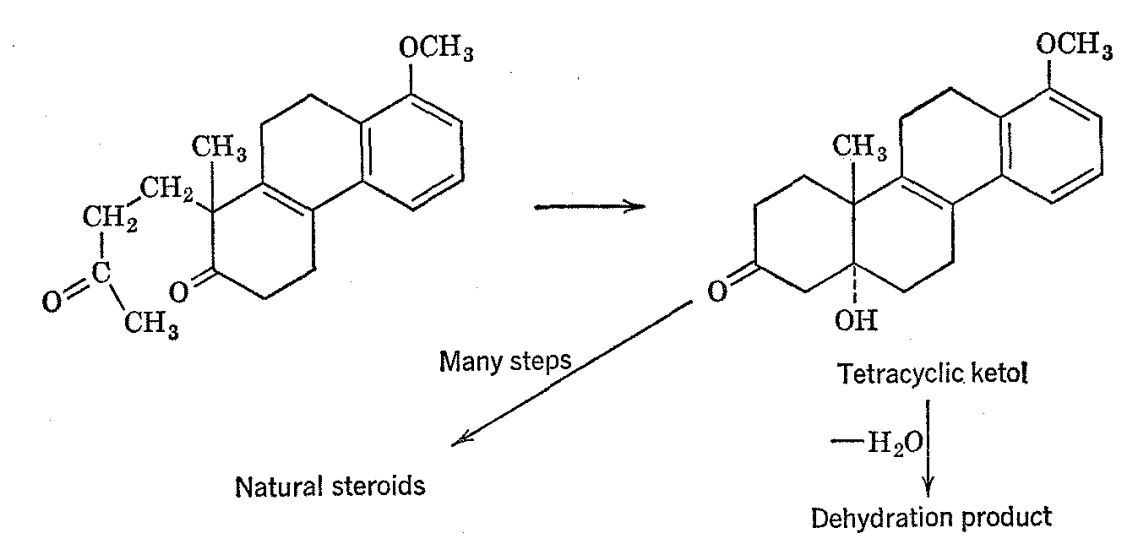
A dehydration product derived from the ltetol was found to have chemical and physical properties hard to reconcile with the given structure of the starting material. The NMR spcctra of the ketol (as the acetate) and its dehydration product are shown in Fig. 2-1 1 with most of the principal resonance lines identified as belonging to the various structural entities. There are two prominent lines in the spectrum of the ketol which are of the appropriate height and position to correspond to C-CH3 groups. However, only one such absorption is expected on the basis of the postulated structure for the ketol. It is interesting that one of the C-CH3 groups disappears on dehydration.
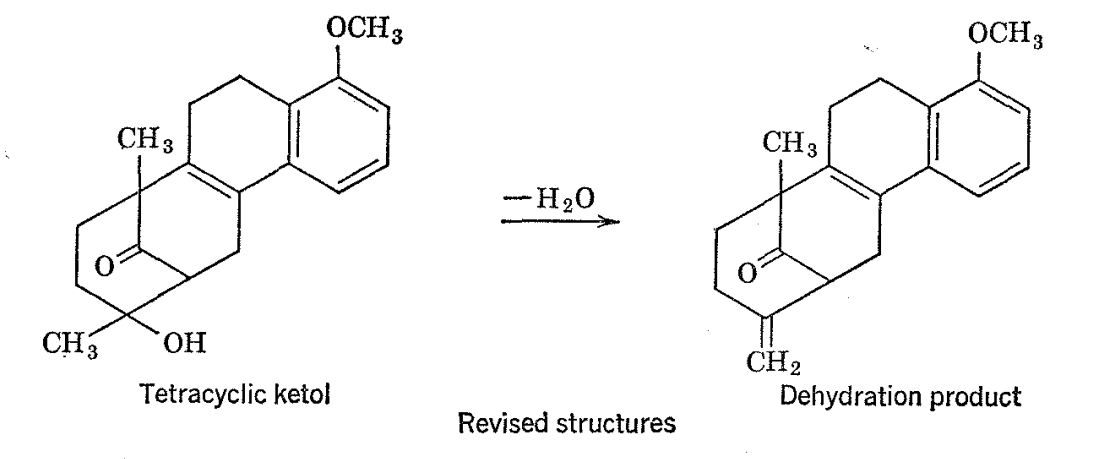
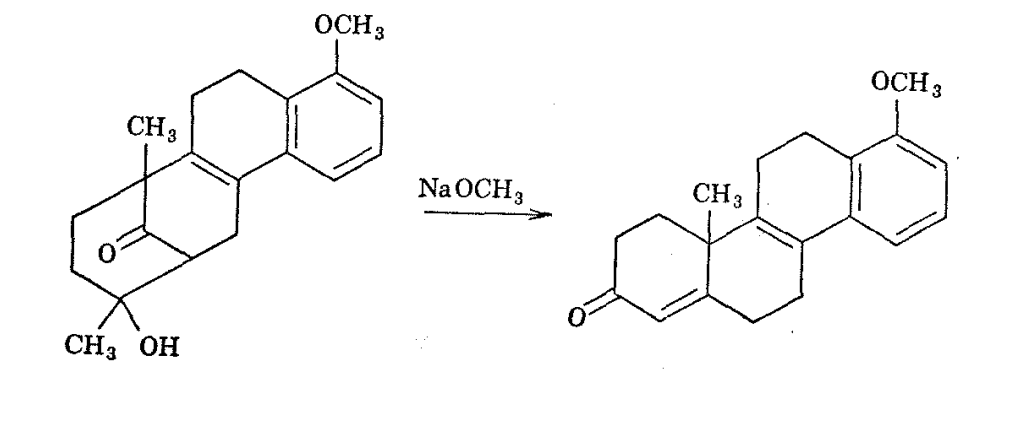
Reevaluation of the structure of the ketol on the basis of its NMR spectra has indicated that it and its dehydration product are best formulated as shown above.19 Synthesis of natural steroidal hormones from the ketol is possible because ring opening, recyclization, and dehydration occur under the influence of strong base.

Nuclear magnetic resonance spectra of a large number of steroidal compounds have been analyzed by Shoolery and Rogers.20 Interesting differences of about 20 cps were noted between axial and equatorial protons at the 3- and 11-positions of the steroid carbon skeleton.
A further elegant example of the use of NMR data for elucidation of complex organic structures is provided by the work of van Tamelen21 on photosantonic acid. Here, a key finding was of a vinyl-proton resonance which could not be accommodated by most of the proposed structures. The spectrum will be discussed further in Chap. 3 in connection with spin-spin splitting, since, besides its characteristic chemical shift, the vinyl proton resonance showed other features which give strong support to van Tamelen's structure for photosantonic acid.
13 F. R. GOSS, C. K. Ing~ld, and J. F. Thorpe, J. Clzem. Soc., 123, 327 (1923).
14 M. G. Ettlinger, J. Am. Chem. Soc., 74, 5805 (1952).
15 A. T. Bottini and J. D. Roberts, J. Org. Chem., 21, 1169 (1956).
16 M. G. Ettlinger and F. Kennedy, Chem. & Znd. (London), 891 (1957).
17 E. F. Silversmith, Y. Kitahara, M. C. Caserio, and J. D. Roberts, 3. Am. Chem. Soc., 80, 5840 (1958).
18 W. S. Johnson and coworkers, J. Am. Chem. Soc., 78, 6302 (1956).
19 Private communication from W. S. Johnson.
20 J. N. Shoolery and M. T. Rogers, I. Am. Chem. Soc., 80, 5121 (1958).
21 E. E. van Tamelen, S. H. Levin, G. Brenner, J. Wolinsky, and P. Aldrich, J. Am. Chem. Soc., 80, 501 (1958).


