12.14: More NMR Examples
- Last updated
- Save as PDF
- Page ID
- 424260
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Additional NMR Examples
For each molecule, predict the number of signals in the 1H-NMR and the 13C-NMR spectra (do not count split peaks - eg. a quartet counts as only one signal). Assume that diastereotopic groups are non-equivalent.
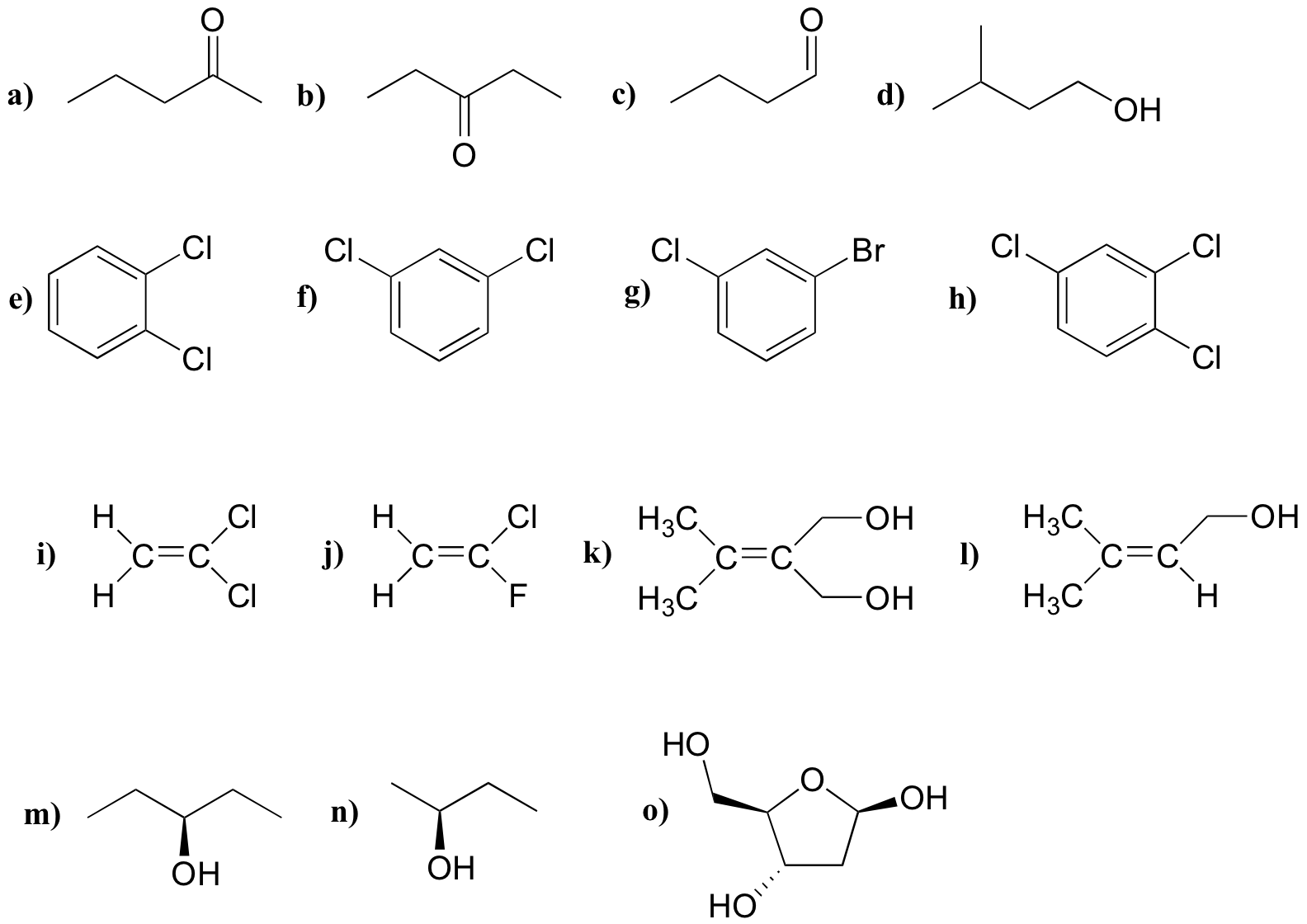
P5.2: For each of the 20 common amino acids, predict the number of signals in the proton-decoupled 13C-NMR spectrum.
P5.3: Calculate the chemical shift value (expressed in Hz, to one decimal place) of each sub-peak on the 1H-NMR doublet signal below. Do this for:
a) a spectrum obtained on a 300 MHz instrument
b) a spectrum obtained on a 100 MHz instrument
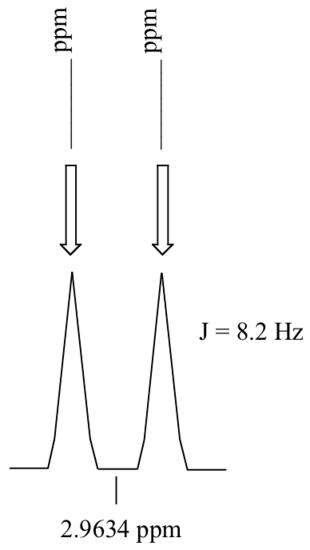
P5.4: Consider a quartet signal in an 1H-NMR spectrum obtained on a 300 MHz instrument. The chemical shift is recorded as 1.7562 ppm, and the coupling constant is J = 7.6 Hz. What is the chemical shift, expressed to the nearest 0.1 Hz, of the furthest downfield sub-peak in the quartet? What is the resonance frequency (again expressed in Hz) of this sub-peak?)
P5.5: One easily recognizable splitting pattern for the aromatic proton signals from disubstituted benzene structures is a pair of doublets. Does this pattern indicate ortho, meta, or para substitution?
P5.6 :Match spectra below to their corresponding structures A-F.
Structures:
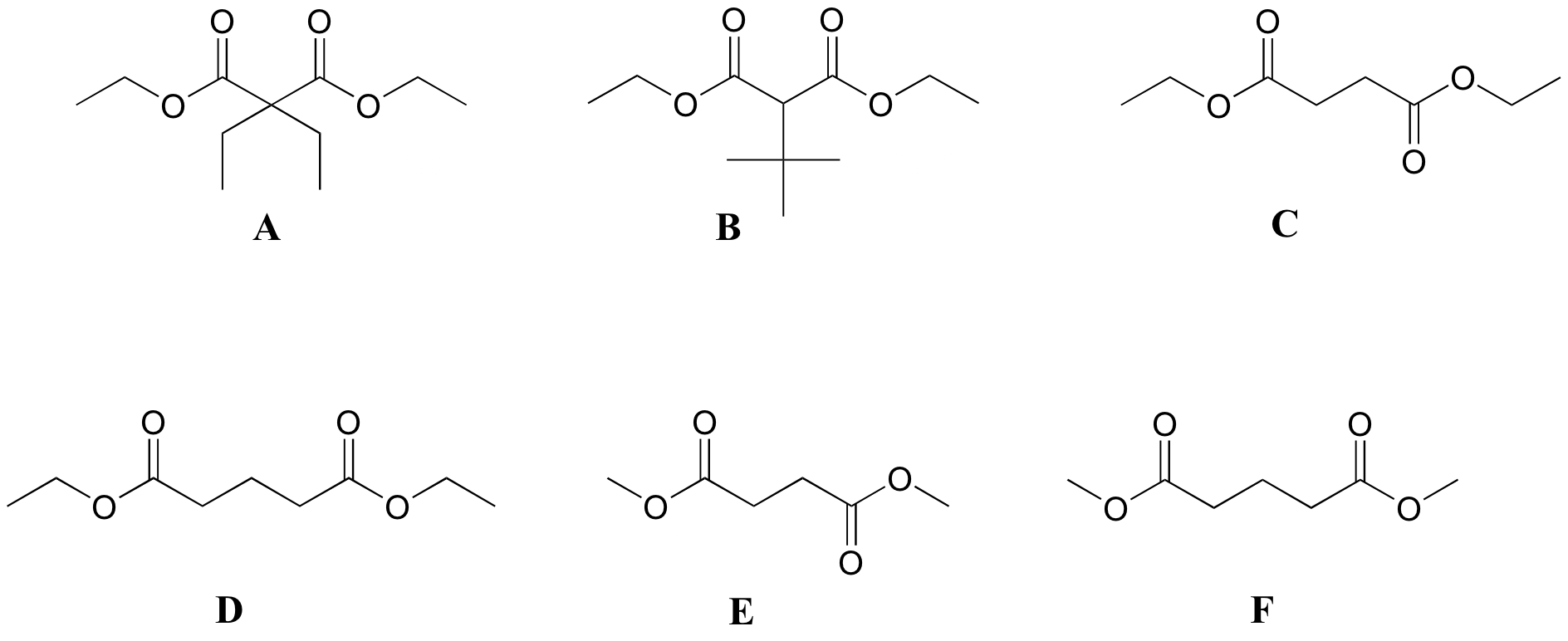
Spectrum 1
|
δ |
splitting |
integration |
|
4.13 |
q |
2 |
|
2.45 |
t |
2 |
|
1.94 |
quintet |
1 |
|
1.27 |
t |
3 |
Spectrum 2
|
δ |
splitting |
integration |
|
3.68 |
s |
3 |
|
2.99 |
t |
2 |
|
1.95 |
quintet |
1 |
Spectrum 3
|
δ |
splitting |
integration |
|
4.14 |
q |
1 |
|
2.62 |
s |
1 |
|
1.26 |
t |
1.5 |
Spectrum 4
|
δ |
splitting |
integration |
|
4.14 |
q |
4 |
|
3.22 |
s |
1 |
|
1.27 |
t |
6 |
|
1.13 |
s |
9 |
Spectrum 5
|
δ |
splitting |
integration |
|
4.18 |
q |
1 |
|
1.92 |
q |
1 |
|
1.23 |
t |
1.5 |
|
0.81 |
t |
1.5 |
Spectrum 6
|
δ |
splitting |
integration |
|
3.69 |
s |
1.5 |
|
2.63 |
s |
1 |
P5.7: Match spectra 7-12 below to their corresponding structures G-L .
Structures:
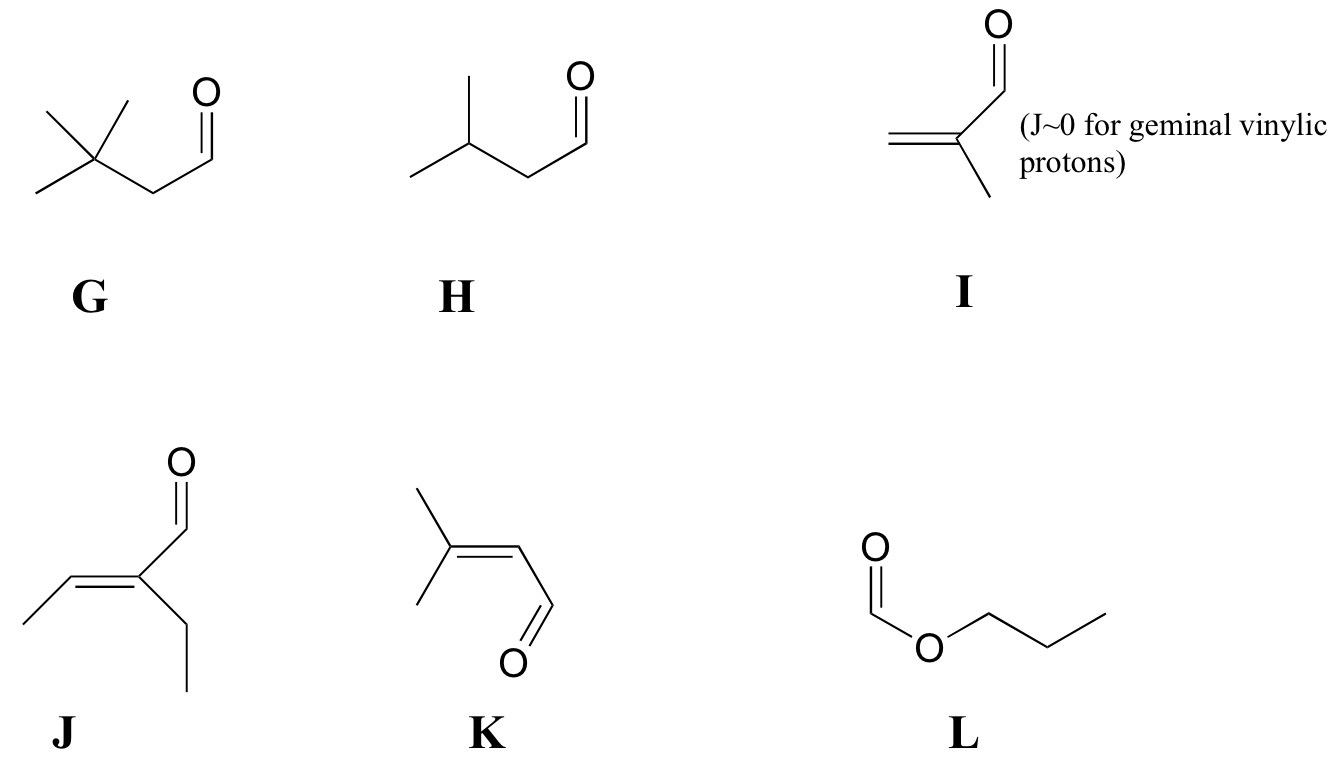
Spectrum 7:
| δ |
splitting |
integration |
|
9.96 |
d |
1 |
|
5.88 |
d |
1 |
|
2.17 |
s |
3 |
|
1.98 |
s |
3 |
Spectrum 8:
|
δ |
splitting |
integration |
|
9.36 |
s |
1 |
|
6.55 |
q |
1 |
|
2.26 |
q |
2 |
|
1.99 |
d |
3 |
|
0.96 |
t |
3 |
Spectrum 9:
|
δ |
splitting |
integration |
|
9.57 |
s |
1 |
|
6.30 |
s |
1 |
|
6.00 |
s |
1 |
|
1.84 |
s |
3 |
Spectrum 10:
|
δ |
splitting |
integration |
|
9.83 |
t |
1 |
|
2.27 |
d |
2 |
|
1.07 |
s |
9 |
Spectrum 11:
|
δ |
splitting |
integration |
|
9.75 |
t |
1 |
|
2.30 |
dd |
2 |
|
2.21 |
m |
1 |
|
0.98 |
d |
6 |
Spectrum 12:
|
δ |
splitting |
integration |
|
8.08 |
s |
1 |
|
4.13 |
t |
2 |
|
1.70 |
m |
2 |
|
0.96 |
t |
3 |
P5.8: Match the 1H-NMR spectra 13-18 below to their corresponding structures M-R .
Structures:
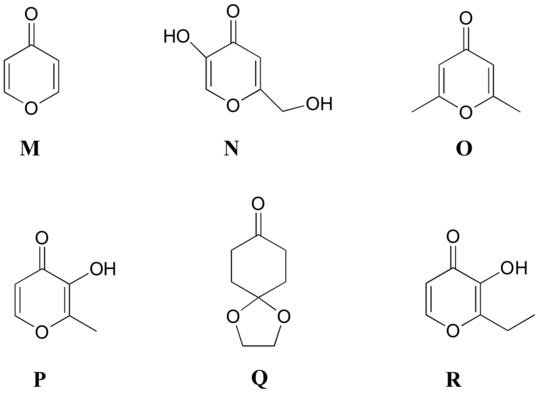
Spectrum 13:
|
δ |
splitting |
integration |
|
8.15 |
d |
1 |
|
6.33 |
d |
1 |
Spectrum 14: 1-723C (structure O)
|
δ |
splitting |
integration |
|
6.05 |
s |
1 |
|
2.24 |
s |
3 |
Spectrum 15:
|
δ |
splitting |
integration |
|
8.57 |
s (b) |
1 |
|
7.89 |
d |
1 |
|
6.30 |
d |
1 |
|
2.28 |
s |
3 |
Spectrum 16:
|
δ |
splitting |
integration |
|
9.05 |
s (b) |
1 |
|
8.03 |
s |
1 |
|
6.34 |
s |
1 |
|
5.68 |
s (b) |
1 |
|
4.31 |
s |
2 |
Spectrum 17:
|
δ |
splitting |
integration |
|
7.76 |
d |
1 |
|
7.57 |
s (b) |
1 |
|
6.44 |
d |
1 |
|
2.78 |
q |
2 |
|
1.25 |
t |
3 |
Spectrum 18:
|
δ |
splitting |
integration |
|
4.03 |
s |
1 |
|
2.51 |
t |
1 |
|
2.02 |
t |
1 |
P5.9: Match the 1H-NMR spectra 19-24 below to their corresponding structures S-X.
Structures:
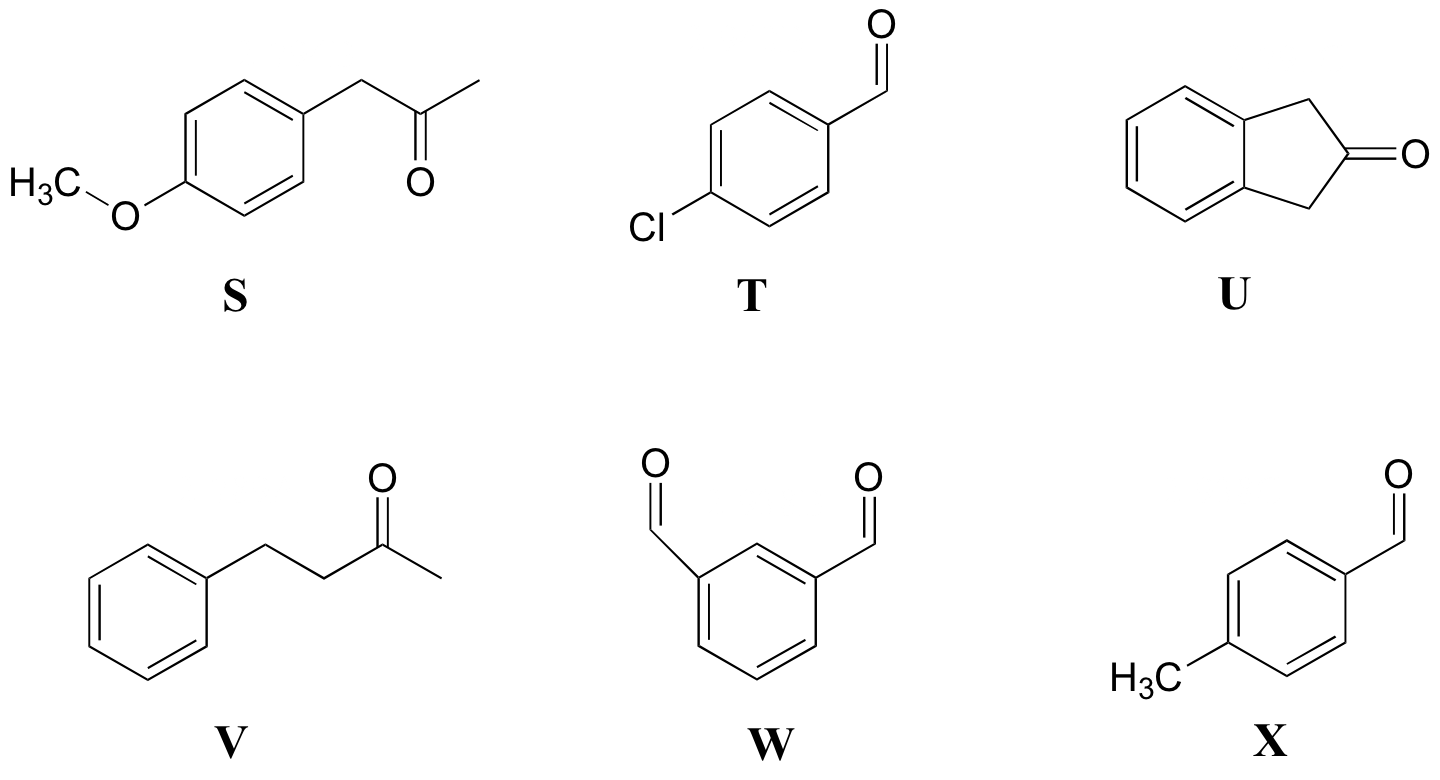
Spectrum 19:
|
δ |
splitting |
integration |
|
9.94 |
s |
1 |
|
7.77 |
d |
2 |
|
7.31 |
d |
2 |
|
2.43 |
s |
3 |
Spectrum 20:
|
δ |
splitting |
integration |
|
10.14 |
s |
2 |
|
8.38 |
s |
1 |
|
8.17 |
d |
2 |
|
7.75 |
t |
1 |
Spectrum 21:
|
δ |
splitting |
integration |
|
9.98 |
s |
1 |
|
7.81 |
d |
2 |
|
7.50 |
d |
2 |
Spectrum 22:
|
δ |
splitting |
integration |
|
7.15-7.29 |
m |
2.5 |
|
2.86 |
t |
1 |
|
2.73 |
t |
1 |
|
2.12 |
s |
1.5 |
Spectrum 23:
|
δ |
splitting |
integration |
|
7.10 |
d |
1 |
|
6.86 |
d |
1 |
|
3.78 |
s |
1.5 |
|
3.61 |
s |
1 |
|
2.12 |
s |
1.5 |
Spectrum 24:
|
δ |
splitting |
integration |
|
7.23-7.30 |
m |
1 |
|
3.53 |
s |
1 |
P5.10: Match the 1H-NMR spectra 25-30 below to their corresponding structures AA-FF.
Structures:
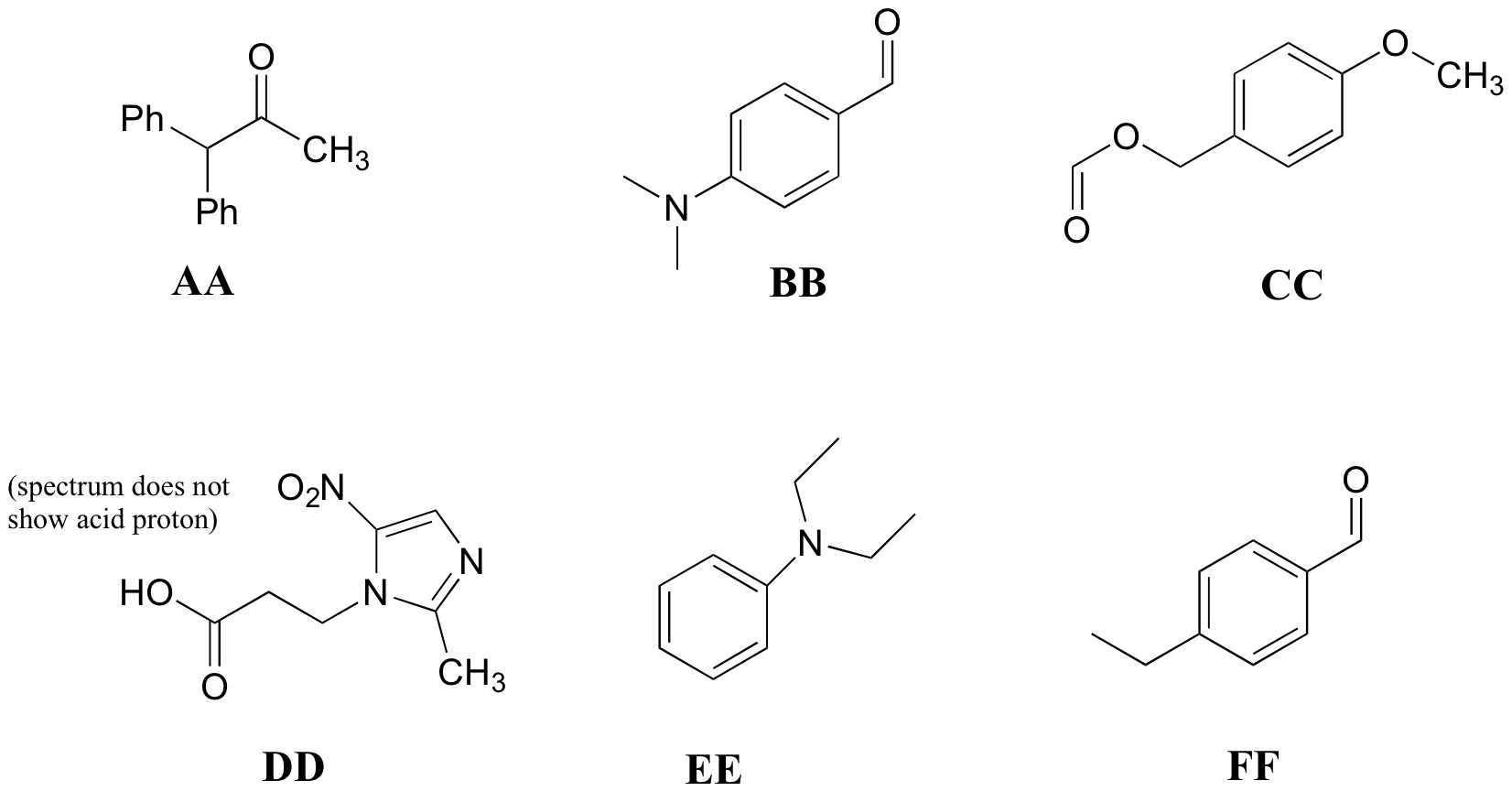
Spectrum 25:
|
δ |
splitting |
integration |
|
9.96 |
s |
1 |
|
7.79 |
d |
2 |
|
7.33 |
d |
2 |
|
2.72 |
q |
2 |
|
1.24 |
t |
3 |
Spectrum 26:
|
δ |
splitting |
integration |
|
9.73 |
s |
1 |
|
7.71 |
d |
2 |
|
6.68 |
d |
2 |
|
3.06 |
s |
6 |
Spectrum 27:
|
δ |
splitting |
integration |
|
7.20-7.35 |
m |
10 |
|
5.12 |
s |
1 |
|
2.22 |
s |
3 |
Spectrum 28:
|
δ |
splitting |
integration |
|
8.08 |
s |
1 |
|
7.29 |
d |
2 |
|
6.87 |
d |
2 |
|
5.11 |
s |
2 |
|
3.78 |
s |
3 |
Spectrum 29:
|
δ |
splitting |
integration |
|
7.18 |
d |
1 |
|
6.65 |
m |
1.5 |
|
3.2 |
q |
2 |
|
1.13 |
t |
3 |
Spectrum 30:
|
δ |
splitting |
integration |
|
8.32 |
s |
1 |
|
4.19 |
t |
2 |
|
2.83 |
t |
2 |
|
2.40 |
s |
3 |
P5.11: Match the 1H-NMR spectra 31-36 below to their corresponding structures GG-LL
Structures:
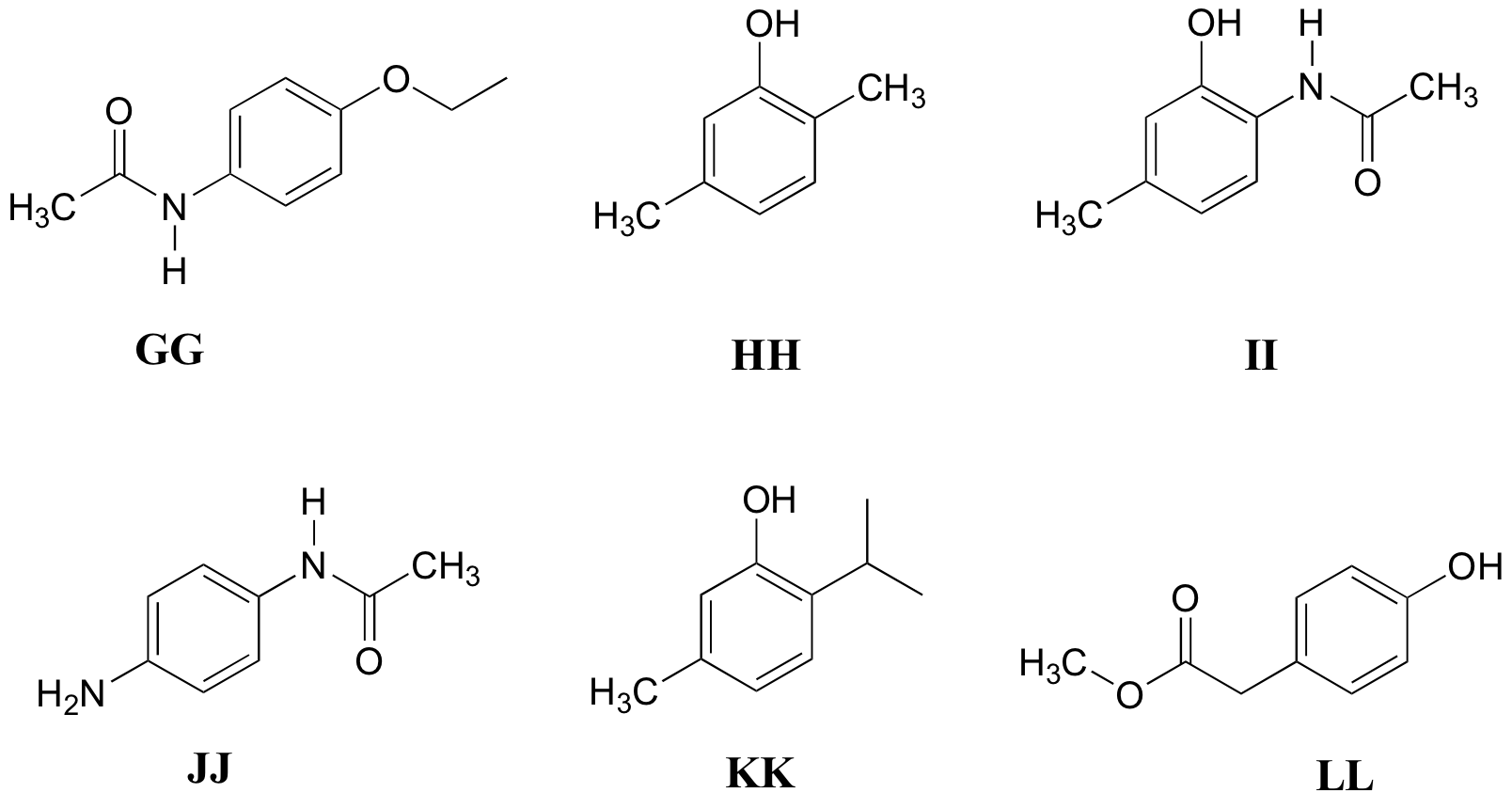
Spectrum 31:
|
δ |
splitting |
integration |
|
6.98 |
d |
1 |
|
6.64 |
d |
1 |
|
6.54 |
s |
1 |
|
4.95 |
s |
1 |
|
2.23 |
s |
3 |
|
2.17 |
s |
3 |
Spectrum 32:
|
δ |
splitting |
integration |
|
7.08 |
d |
1 |
|
6.72 |
d |
1 |
|
6.53 |
s |
1 |
|
4.81 |
s |
1 |
|
3.15 |
7-tet |
1 |
|
2.24 |
s |
3 |
|
1.22 |
d |
6 |
Spectrum 33:
|
δ |
splitting |
integration |
|
7.08 |
d |
2 |
|
6.71 |
d |
2 |
|
6.54 |
s |
1 |
|
3.69 |
s |
3 |
|
3.54 |
s |
2 |
Spectrum 34:
|
δ |
splitting |
integration |
|
9.63 |
s |
1 |
|
7.45 |
d |
2 |
|
6.77 |
d |
2 |
|
3.95 |
q |
2 |
|
2.05 |
s |
3 |
|
1.33 |
t |
3 |
Spectrum 35:
|
δ |
splitting |
integration |
|
9.49 |
s |
1 |
|
7.20 |
d |
2 |
|
6.49 |
d |
2 |
|
4.82 |
s |
2 |
|
1.963 |
s |
3 |
Spectrum 36:
|
δ |
splitting |
integration |
|
9.58 |
s(b) |
1 |
|
9.31 |
s |
1 |
|
7.36 |
d |
1 |
|
6.67 |
s |
1 |
|
6.55 |
d |
1 |
|
2.21 |
s |
3 |
|
2.11 |
s |
3 |
P5.12: Use the NMR data given to deduce structures.
a ) Molecular formula: C5H8O
1H-NMR:
|
δ |
splitting |
integration |
|
9.56 |
s |
1 |
|
6.25 |
d (J~1 Hz) |
1 |
|
5.99 |
d (J~1 Hz) |
1 |
|
2.27 |
q |
2 |
|
1.18 |
t |
3 |
13C-NMR
|
δ |
DEPT |
|
194.60 |
CH |
|
151.77 |
C |
|
132.99 |
CH2 |
|
20.91 |
CH2 |
|
11.92 |
CH3 |
b) Molecular formula: C7H14O2
1H-NMR:
|
δ |
splitting |
integration |
|
3.85 |
d |
2 |
|
2.32 |
q |
2 |
|
1.93 |
m |
1 |
|
1.14 |
t |
3 |
|
0.94 |
d |
6 |
13C-NMR
|
δ |
DEPT |
|
174.47 |
C |
|
70.41 |
CH2 |
|
27.77 |
CH |
|
27.64 |
CH2 |
|
19.09 |
CH3 |
|
9.21 |
CH3 |
c) Molecular formula: C5H12O
1H-NMR:
|
δ |
splitting |
integration |
|
3.38 |
s |
2H |
|
2.17 |
s |
1H |
|
0.91 |
s |
9H |
13C-NMR
|
δ |
DEPT |
|
73.35 |
CH2 |
|
32.61 |
C |
|
26.04 |
CH3 |
d) Molecular formula: C10H12O
1H-NMR:
|
δ |
splitting |
integration |
|
7.18-7.35 |
m |
2.5 |
|
3.66 |
s |
1 |
|
2.44 |
q |
1 |
|
1.01 |
t |
1.5 |
13C-NMR
|
δ |
DEPT |
|
208.79 |
C |
|
134.43 |
C |
|
129.31 |
CH |
|
128.61 |
CH |
|
126.86 |
CH |
|
49.77 |
CH2 |
|
35.16 |
CH2 |
|
7.75 |
CH3 |
P5.13:
13C-NMR data is given for the molecules shown below. Complete the peak assignment column of each NMR data table.
a)
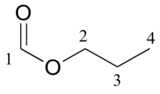
|
δ |
DEPT |
carbon # |
|
161.12 |
CH |
|
|
65.54 |
CH2 |
|
|
21.98 |
CH2 |
|
|
10.31 |
CH3 |
b)
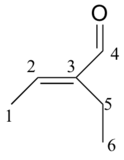
|
δ |
DEPT |
carbon # |
|
194.72 |
C |
|
|
149.10 |
C |
|
|
146.33 |
CH |
|
|
16.93 |
CH2 |
|
|
14.47 |
CH3 |
|
|
12.93 |
CH3 |
c)
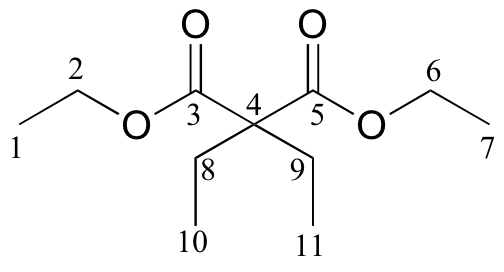
|
δ |
DEPT |
carbon # |
|
171.76 |
C |
|
|
60.87 |
CH2 |
|
|
58.36 |
C |
|
|
24.66 |
CH2 |
|
|
14.14 |
CH3 |
|
|
8.35 |
CH3 |
d)
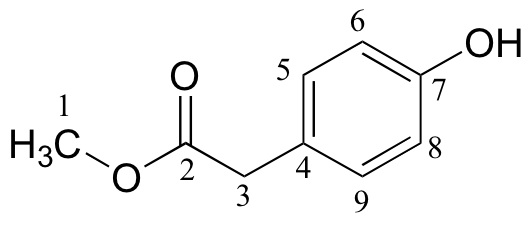
|
δ |
DEPT |
carbon # |
|
173.45 |
C |
|
|
155.01 |
C |
|
|
130.34 |
CH |
|
|
125.34 |
C |
|
|
115.56 |
CH |
|
|
52.27 |
CH3 |
|
|
40.27 |
CH2 |
e)
|
δ |
DEPT |
carbon # |
|
147.79 |
C |
|
|
129.18 |
CH |
|
|
115.36 |
CH |
|
|
111.89 |
CH |
|
|
44.29 |
CH2 |
|
|
12.57 |
CH3 |
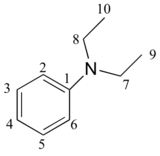
P5.14: You obtain the following data for an unknown sample. Deduce its structure.
1H-NMR:
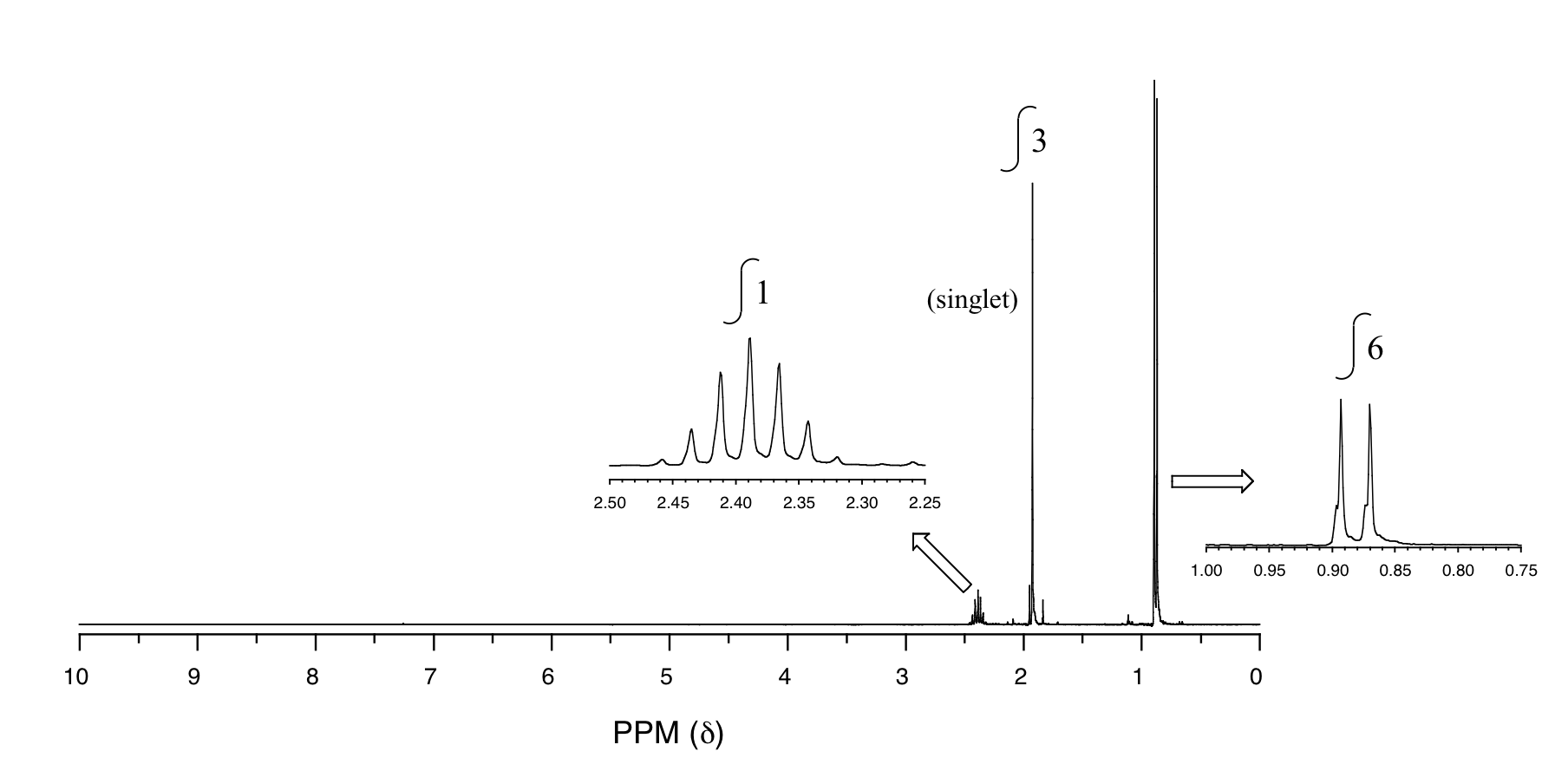
13C-NMR:
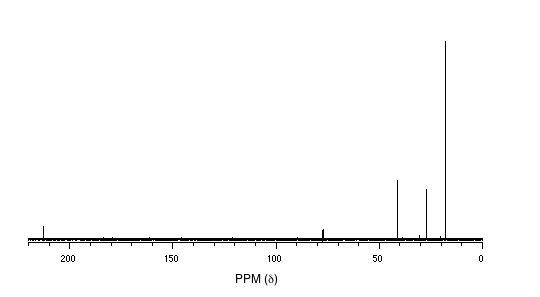
Mass Spectrometry:
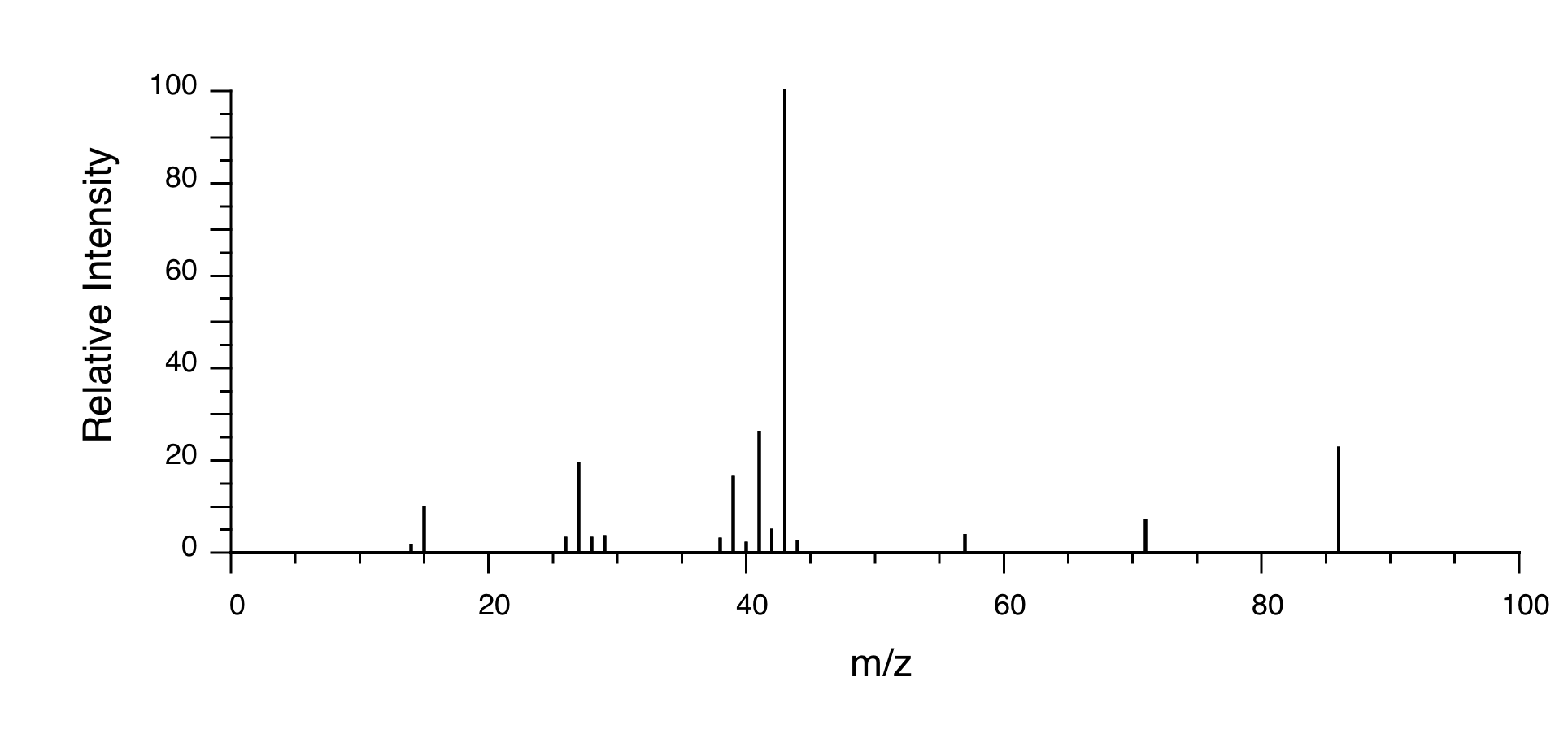
P5.15:You take a 1H-NMR spectrum of a sample that comes from a bottle of 1-bromopropane. However, you suspect that the bottle might be contaminated with 2-bromopropane. The NMR spectrum shows the following peaks:
|
δ |
splitting |
integration |
|
4.3 |
septet |
0.0735 |
|
3.4 |
triplet |
0.661 |
|
1.9 |
sextet |
0.665 |
|
1.7 |
doublet |
0.441 |
|
1.0 |
triplet |
1.00 |
How badly is the bottle contaminated? Specifically, what percent of the molecules in the bottle are 2-bromopropane?
Challenge problems
C5.1: All of the 13C-NMR spectra shown in this chapter include a signal due to CDCl3, the solvent used in each case. Explain the splitting pattern for this signal.
C5.2: Researchers wanted to investigate a reaction which can be catalyzed by the enzyme alcohol dehydrogenase in yeast. They treated 4'-acylpyridine (1) with living yeast, and isolated the alcohol product(s) (some combination of 2A and 2B).
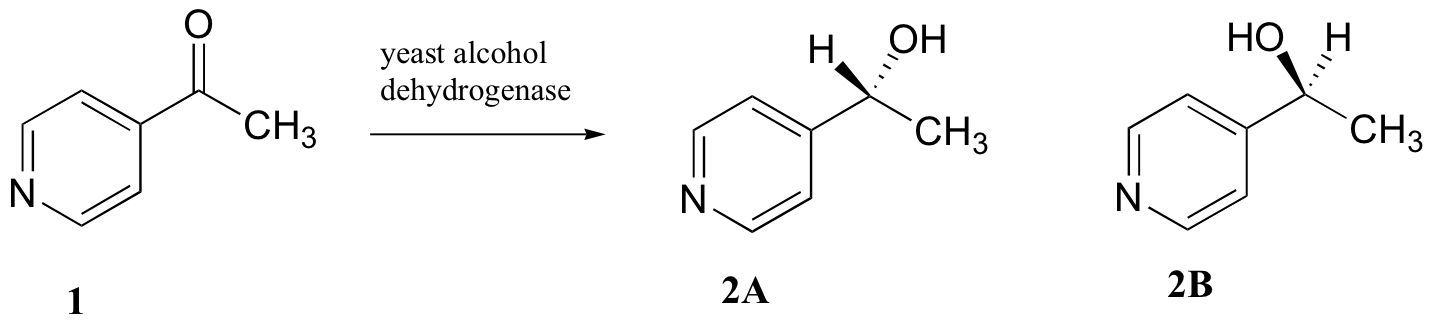
a) Will the products 2A and 2B have identical or different 1H-NMR spectra? Explain.
b) Suggest a 1H-NMR experiment that could be used to determine what percent of starting material (1) got turned into product (2A and 2B).
c) With purified 2A/2B, the researchers carried out the subsequent reaction shown below to make 3A and 3B, known as 'Mosher's esters'. Do 3A and 3B have identical or different 1H-NMR spectra? Explain.
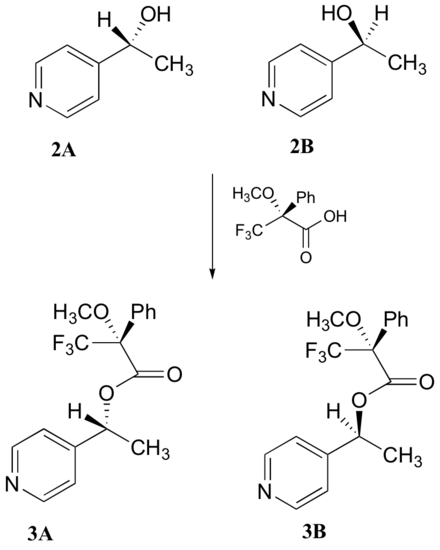
d) Explain, very specifically, how the researchers could use 1H-NMR to determine the relative amounts of 2A and 2B formed in the reaction catalyzed by yeast enzyme.

