22.6: Ester Chemistry
- Page ID
- 45953
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction
Esters are readily synthesized and naturally abundant. Esters are frequently the source of flavors and aromas in many fruits and flowers.
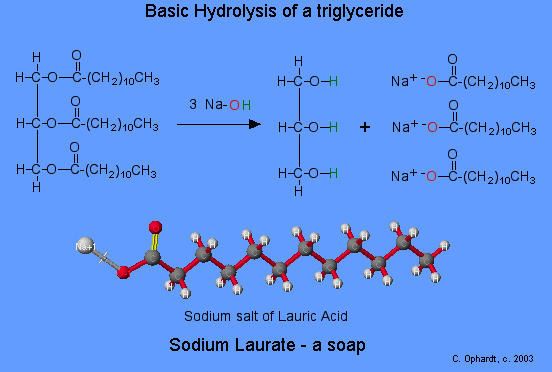
Esters also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). Soap is produced by a saponification (basic hydrolysis) reaction of a fat or oil.
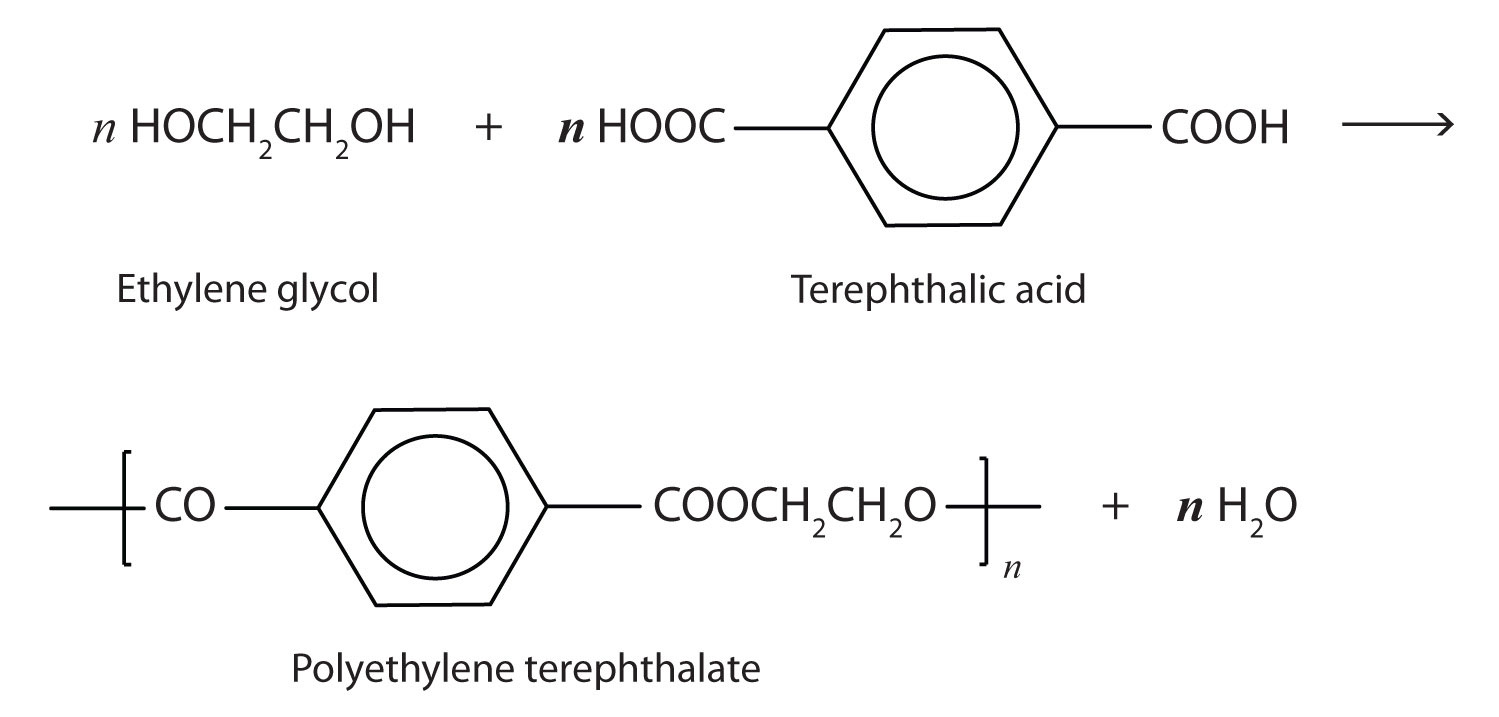
Esters are also present in a number of important biological molecules and have several commercial and synthetic application. For example, polyester molecules make excellent fibers and are used in many fabrics. A knitted polyester tube, which is biologically inert, can be used in surgery to repair or replace diseased sections of blood vessels. PET is used to make bottles for soda pop and other beverages. It is also formed into films called Mylar. When magnetically coated, Mylar tape is used in audio- and videocassettes. Synthetic arteries can be made from PET, polytetrafluoroethylene, and other polymers.
The most important polyester, polyethylene terephthalate (PET), is made from terephthalic acid and ethylene glycol monomers:
Synthesis of Esters
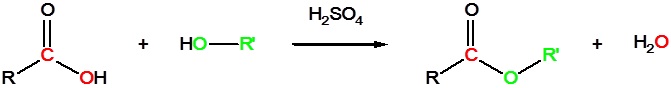
Carboxylic acids can react with alcohols to form esters in the presence of an acid catalyst as shown in the reaction below.
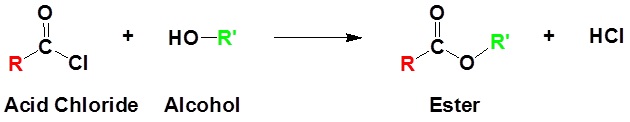
Acid chlorides react with alcohols to form esters as shown in the reaction below.
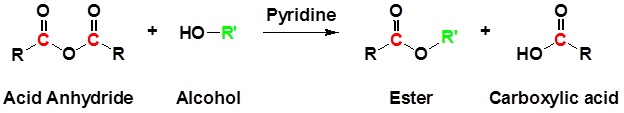
Acid anhydrides also react with alcohols to form esters as shown in the reaction below.
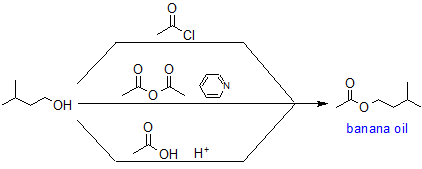
As an example, the synthesis of banana oil (isoamyl acetate) is an example of these two reactions.
Example: Ester Synthesis
Esters can be also be synthesized from trans-esterification reactions. Trans-esterification is discussed in the next section on Reactivity of Esters
Reactivity of Esters
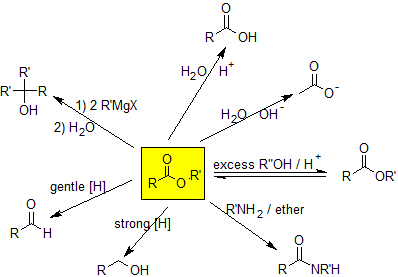
Esters can be hydrolyzed to carboxylic acids under acidic or basic conditions. Basic hydrolysis can be used to convert fats and oils into soap and is called a saponification reaction. Esters can be converted to amides via an aminolysis reaction. Esters can undergo trans-esterification reactions to form different esters by applying LeChatlier's principle to this equilibrium reaction. Esters can be reduced to form alcohols or aldehydes depending on the reducing agent. Esters also react with organometallic compounds to form tertiary alcohols. The reaction map for esters is shown below.
Ester Hydrolysis
Ester hydrolysis requires an acid catalyst or base promotion to occur. Esters are less reactive than acyl halides and acid anhydrides because the alkoxide group is a poor leaving group with its negative charge fully localized on a single oxygen atom.
Ester Hydrolysis - Acid Catalyzed
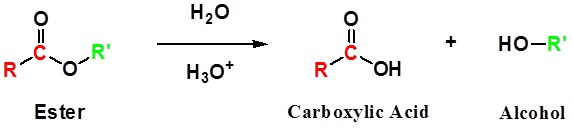
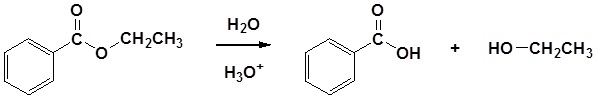
Esters can be cleaved back into a carboxylic acid and an alcohol by reaction with water and a catalytic amount of acid as shown in the reaction below.
The acid catalyzed hydrolysis of ethyl benzoate is shown below as an example.
Example: Acid Catalyzed Ester Hydrolysis
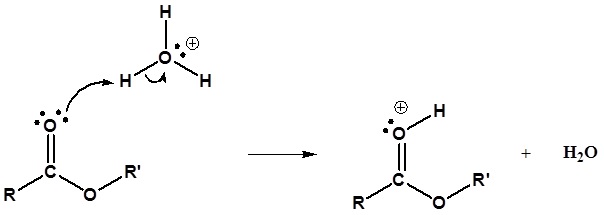
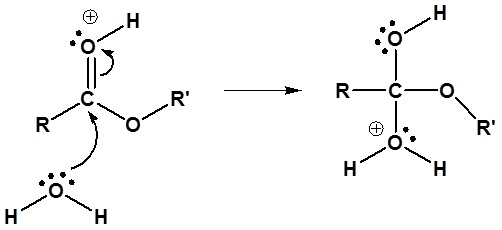
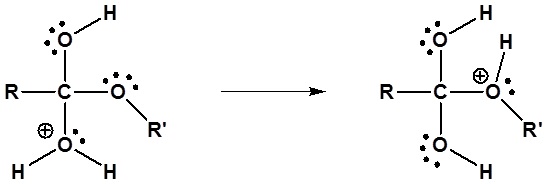
The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. The nucleophilic water reacts with the electrophilic carbonyl carbon atom to form the tetrahedral intermediate. Proton transfer reactions occur to create a good leaving group when the carbonyl reforms. The complete mechanism is shown below.
1) Protonation of the Carbonyl
2) Nucleophilic reaction by water
3) Proton transfer
4) Leaving group removal
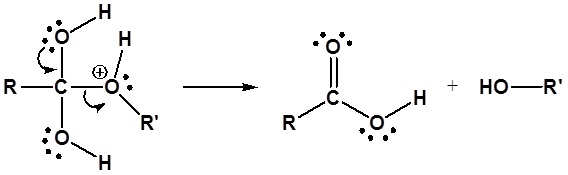
Ester Hydrolysis - Base promoted
Esters can be cleaved back into a carboxylic acid and an alcohol by reaction with water and a base. The reaction is called a saponification from the Latin sapo which means soap. The name comes from the fact that soap used to me made by the ester hydrolysis of fats. Due to the basic conditions, a carboxylate ion is made rather than a carboxylic acid. The hydroxide ions are consumed in the reaction so it is described as "base promoted".
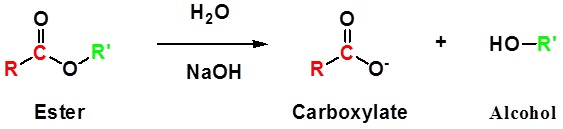

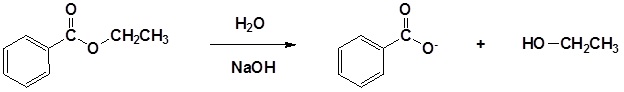
The base promoted hydrolysis of ethyl benzoate is shown below as an example.
Example: Base Promoted Hydrolysis of Esters
The mechanism for the base promoted hydrolysis reaction begins with the nucleophilic hydroxide reacting with the electrophilic carbonyl carbon atom to form the tetrahedral intermediate. The carbonyl reforms with the loss of the alkoxide leaving group. The alkoxide then deprotonates the resulting carboxylic acid. The complete mechanism is shown below.
1) Nucleophilic reaction by hydroxide
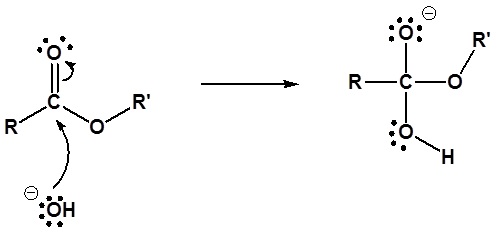
2) Leaving group removal
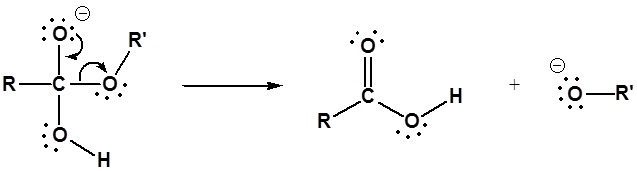
3) Deprotonation
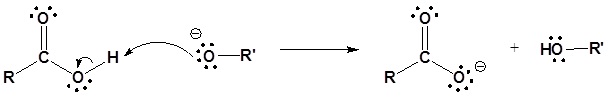
Nuclephilic Acyl Substitution Reactions from Esters
Carboxylic acid derivatives can be synthesized from esters via the nucleophilic acyl substitution mechanism previously discussed.
Ester Synthesis: Trans-Esterification
Trans-esterification is the conversion of a carboxylic acid ester into a different carboxylic acid ester. When an ester is placed in a large excess of an alcohol along with presence of either an acid or a base there can be an exchange of alkoxy groups. The large excess of alcohol is used to drive the reaction forward. The most common method of trans-esterification is the reaction of the ester with an alcohol in the presence of an acid catalyst as shown below.
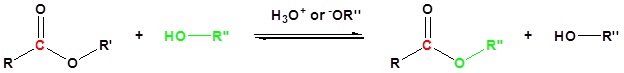
The trans-esterification of ethyl acetate to methyl acetate and methyl benzoate to ethyl benzoate are shown below as examples.
Example: Trans-esterification Reactions
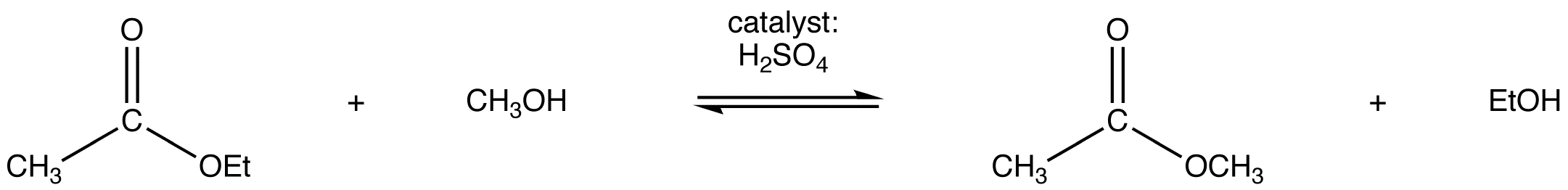
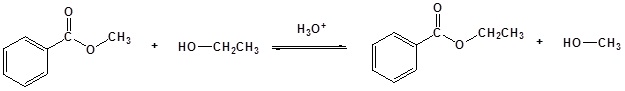
Under acidic conditions, the reaction mechanism begins with protonation of the carbonyl oxygen which increases the reactivity of the ester. An alcohol then reactions with protonated ester to form the tetrahedral intermediate. After several proton transfers, the carbonyl reforms to produce a new ester. The complete mechanism is shown below for the trans-esterification of ethyl actetae to methyl acetate.
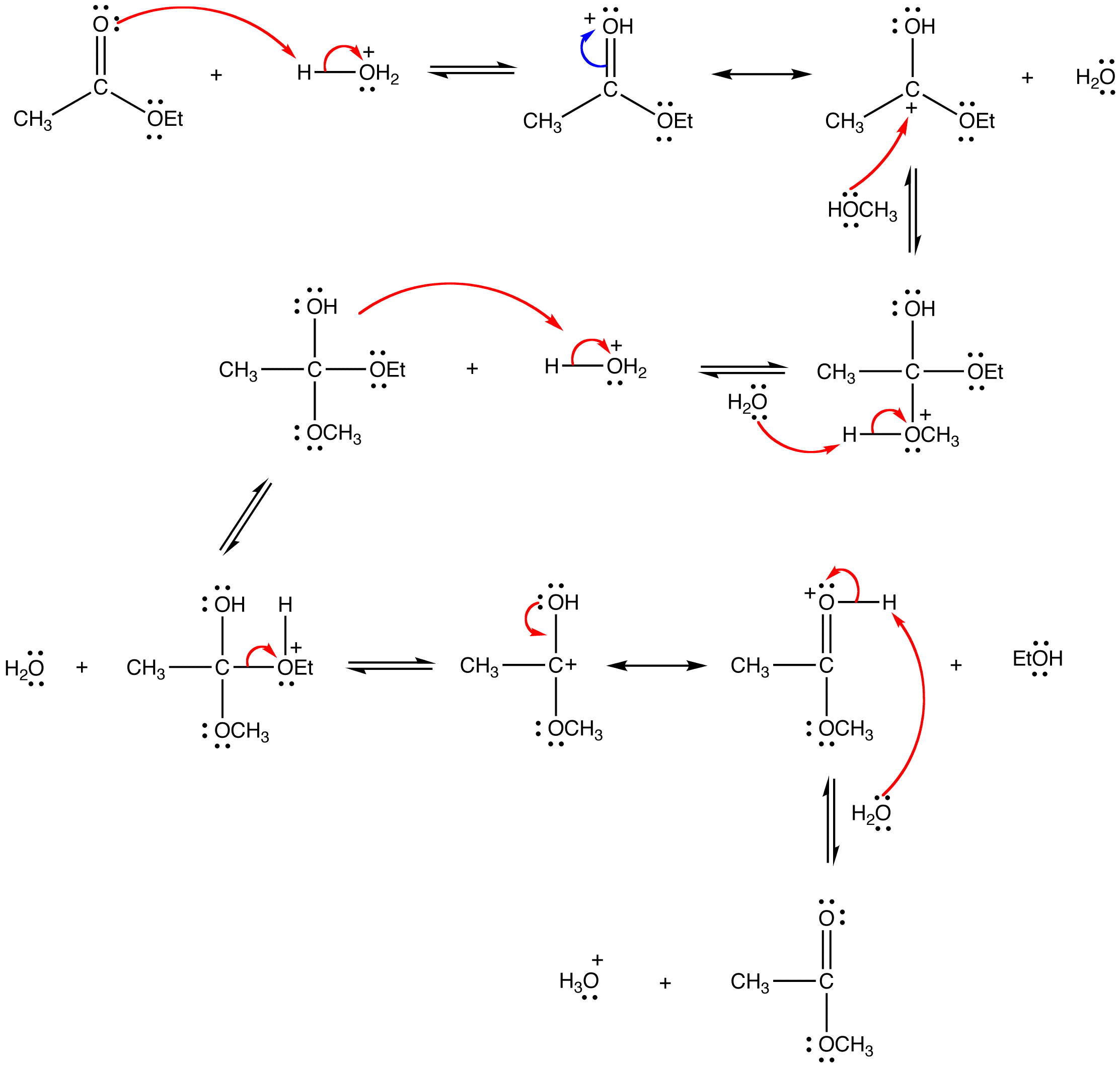
Since both the reactants and the products are an ester and an alcohol, the reaction is reversible and the equilibrium constant is close to one. Consequently, the Le Chatelier’s principle has to be exploited to drive the reaction to completion. The simplest way to do so is to use the alcohol as the solvent as well.
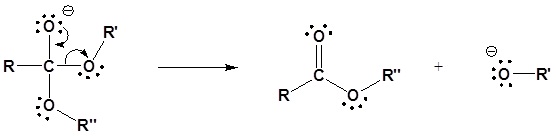
Under basic conditions, the mechanism begins with the nucleophilic reaction of the alkoxide with the carbonyl carbon to produce the tetrahedral intermediate. The carbonyl reforms with the loss of the leaving group to produce a new ester.
1) Nucleophilic reaction by an alkoxide
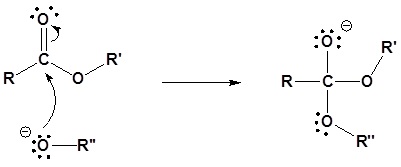
2) Leaving group removal
Aminolysis: Conversion of Esters into Amides
Esters react with ammonia and 1o or 2o alkyl amines to yield amides in a reaction called aminolysis.
The aminolysis of ethyl benzoate is shown below as an example. The mechanism for this reaction is analogous to the base promoted hydrolysis reaction of esters shown above.
Example: Aminolysis of Esters
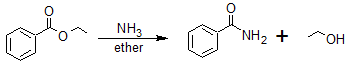
Ester Reduction Reactions
Ester Reduction to a 1o Alcohol
Esters can be converted to 1o alcohols using LiAlH4, while sodium borohydride (NaBH4) is not a strong enough reducing agent to perform this reaction.
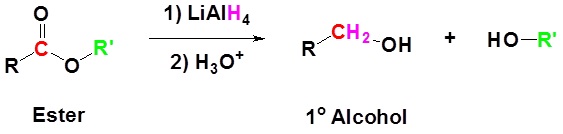
The reduction of ethyl benzoate to benzyl alcohol and ethanol is shown as an example.
Example: Ester Reduction to a 1o Alcohol
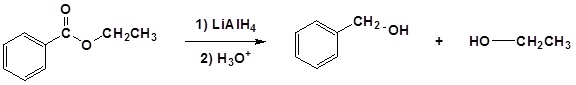
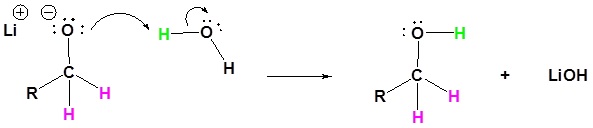
The mechanism begins with a hydride nucleophile reacting with the ester carbonyl carbon to form the tetrahedral intermediate. The carbonyl reforms to produce an aldehyde with the loss of the alkoxide ion. The resulting aldehyde undergoes a subsequent reaction with a hydride nucleophile to form another tetrahedral intermediate. The carbonyl is not able to reform, because there are no stable leaving groups. Therefore, the alkoxide (tetrahedral intermediate) is protonated to produce a primary alcohol. The complete mechanism is shown below.
1) Nucleophilic reaction by the hydride
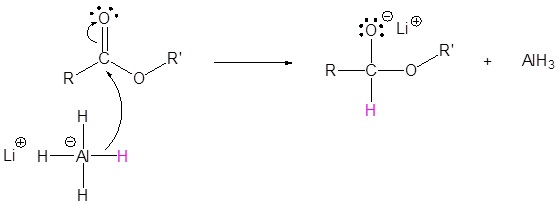
2) Leaving group removal
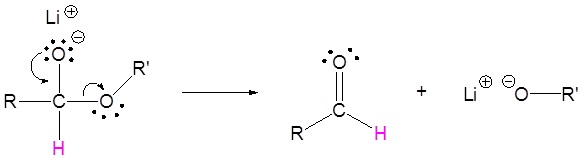
3) Nucleopilic reaction by the hydride anion
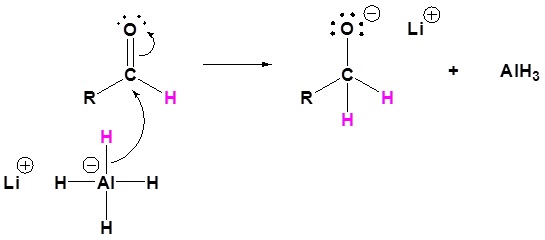
4) The alkoxide is protonated
Ester Reduction to an Aldehyde
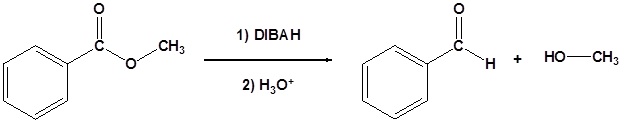
Esters can be converted to aldehydes using diisobutylaluminum hydride (DIBAH). The reaction is usually carried out at -78 oC to prevent reaction with the aldehyde product.
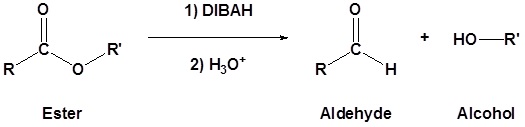
The reduction of methyl benzoate to form benzaldehyde is shown as an example. The mechanism is analogous to the LiAlH4 mechanism shown above with the important difference that the reaction stops after the aldehyde is produced because the DIBAH reducing agent is not strong enough to reduce the aldehyde at low temperatures.
Example: Ester Reduction to an Aldehyde
Ester Reactions with Organometallic Compounds
Grignard reagents
Esters react with Grignard reagents to form tertiary alcohols. This reaction is analogous to the reaction discussed for acid chlorides with Grignard reagents. The first equivalent of the Grignard reagent produces a ketone which reacts with the second equivalent of the Grignard reagent to produce a tertiary alcohol. In effect, the Grignard reagent adds twice as shown in the reaction below.
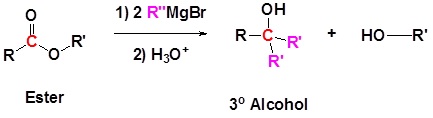
The reaction of methyl benzoate with a Grignard reagent to produce 3-phenyl-3-pentanol.
Example: Ester Reaction with a Grignard Reagent
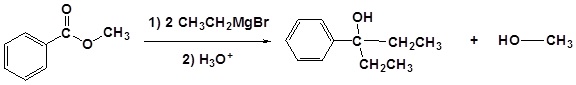
The mechanism begins with a carbide nucleophile from the Grignard reagent reacting with the ester carbonyl carbon to form the tetrahedral intermediate. The carbonyl reforms to produce a ketone with the loss of the alkoxide ion. The resulting ketone undergoes a subsequent reaction with a carbide nucleophile from the Grignard reagent to form another tetrahedral intermediate. The carbonyl is not able to reform, because there are no stable leaving groups. Therefore, the alkoxide (tetrahedral intermediate) is protonated to produce a tertiary alcohol. The complete mechanism is shown below.
1) Nucleophilic reaction
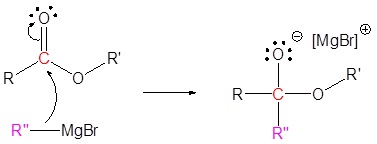
2) Leaving group removal
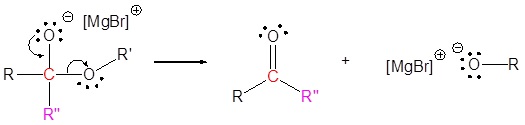
3) Nucleophilic reaction
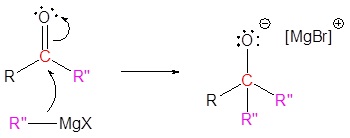
4) Protonation
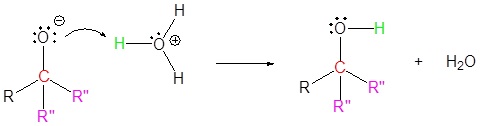
Exercise
8. Why is the alkaline hydrolysis of an ester not a reversible process? Why doesn't the reaction with a hydroxide ion and a carboxylic acid produce an ester?
9. Draw the product of the reaction between the following molecule and LiAlH4, and the product of the reaction between the following molecule and DIBAL.
10. Prepare the following molecules from esters and Grignards?
(a)
(b)
(c)
- Answer
-
8. The reaction between a carboxylic acid and a hydroxide ion is an acid base reaction, which produces water and a carboxylate anion.
9.
10.
(a)
(b)
(c)
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Charles Ophardt (Professor Emeritus, Elmhurst College); Virtual Chembook
- Gamini Gunawardena from the OChemPal site (Utah Valley University)