20.2: Basicity of Amines and Ammonium Salt Formation
- Page ID
- 45608
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Basicity of nitrogen groups
When evaluating the relative basicity of several nitrogen-containing functional groups: amines, amides, anilines, imines, and nitriles, the central question is: how reactive (and thus how basic) is the lone pair on the nitrogen? In other words, how much does that lone pair want to break away from the nitrogen nucleus and form a new bond with a hydrogen?
Comparing the basicity of alkyl amines to ammonia
Alkyl groups donate electrons to the more electronegative nitrogen. This inductive effect makes the electron density on the alkylamine's nitrogen greater than the nitrogen of ammonium. Correspondingly, primary, secondary, and tertiary alkyl amines are more basic than ammonia. Inductive effects are also moderated by the increased steric hindrance of alkyl groups (R grps).
Comparing the basicity of alkylamines to amides
With an alkyl amine the lone pair electron is localized on the nitrogen. However, the lone pair electron on an amide are delocalized between the nitrogen and the oxygen through resonance. This makes amides much less basic compared to alkylamines.
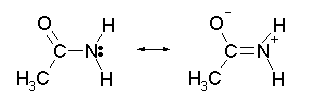
When an amide reacts with an acid, the protonation occurs at the carbonyl oxygen and not the nitrogen. The cation resulting from oxygen protonation is resonance stabilized., while the cation resulting for the protonation of nitrogen is not resonance stabilized.
Basicity of heterocyclic amines
When a nitrogen atom is incorporated directly into an aromatic ring, its basicity depends on the bonding context. In a pyridine ring, for example, the nitrogen lone pair occupies an sp2-hybrid orbital, and is not part of the aromatic sextet - it is essentially an imine nitrogen. Its electron pair is available for forming a bond to a proton, and thus the pyridine nitrogen atom is somewhat basic.
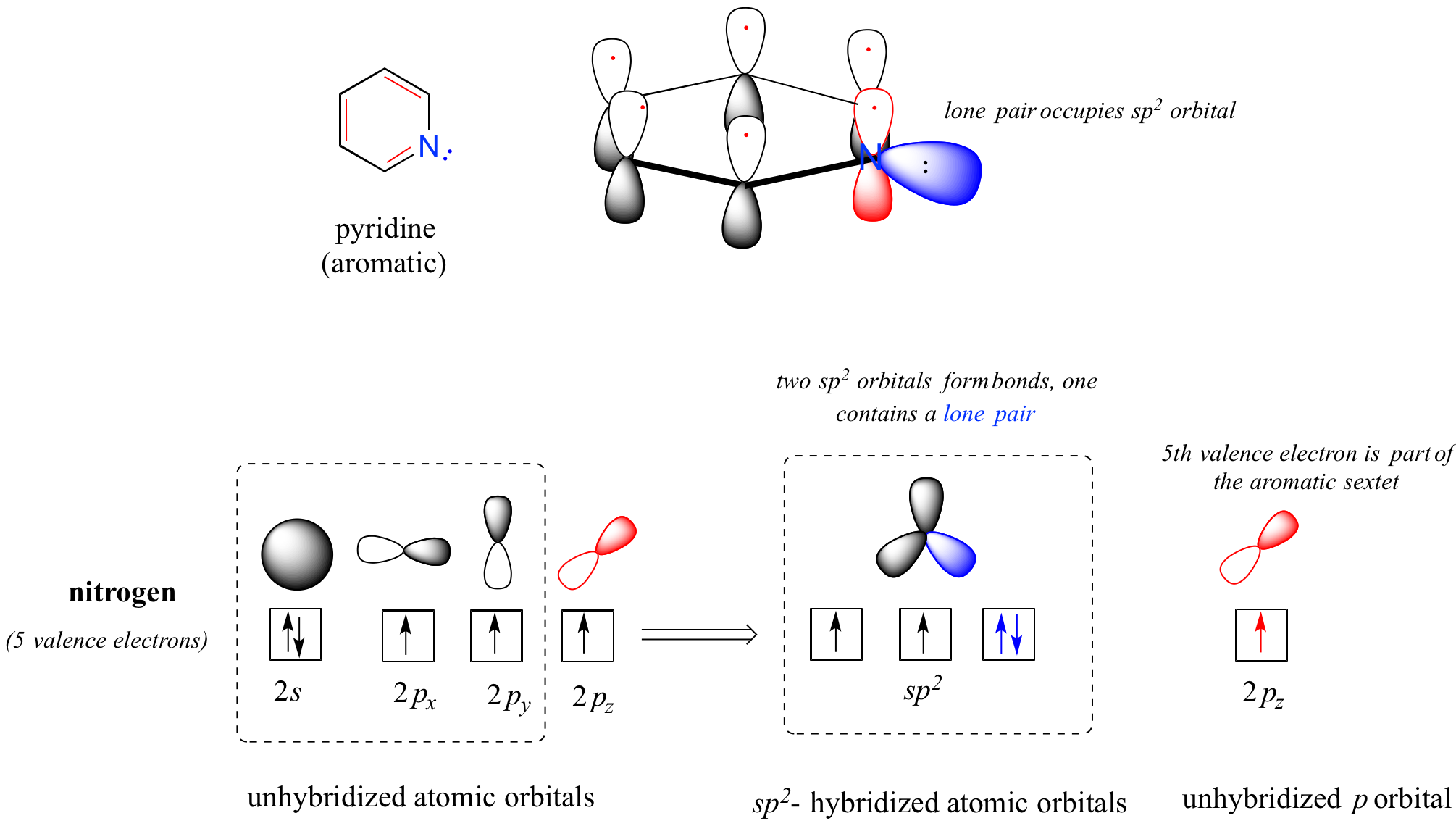
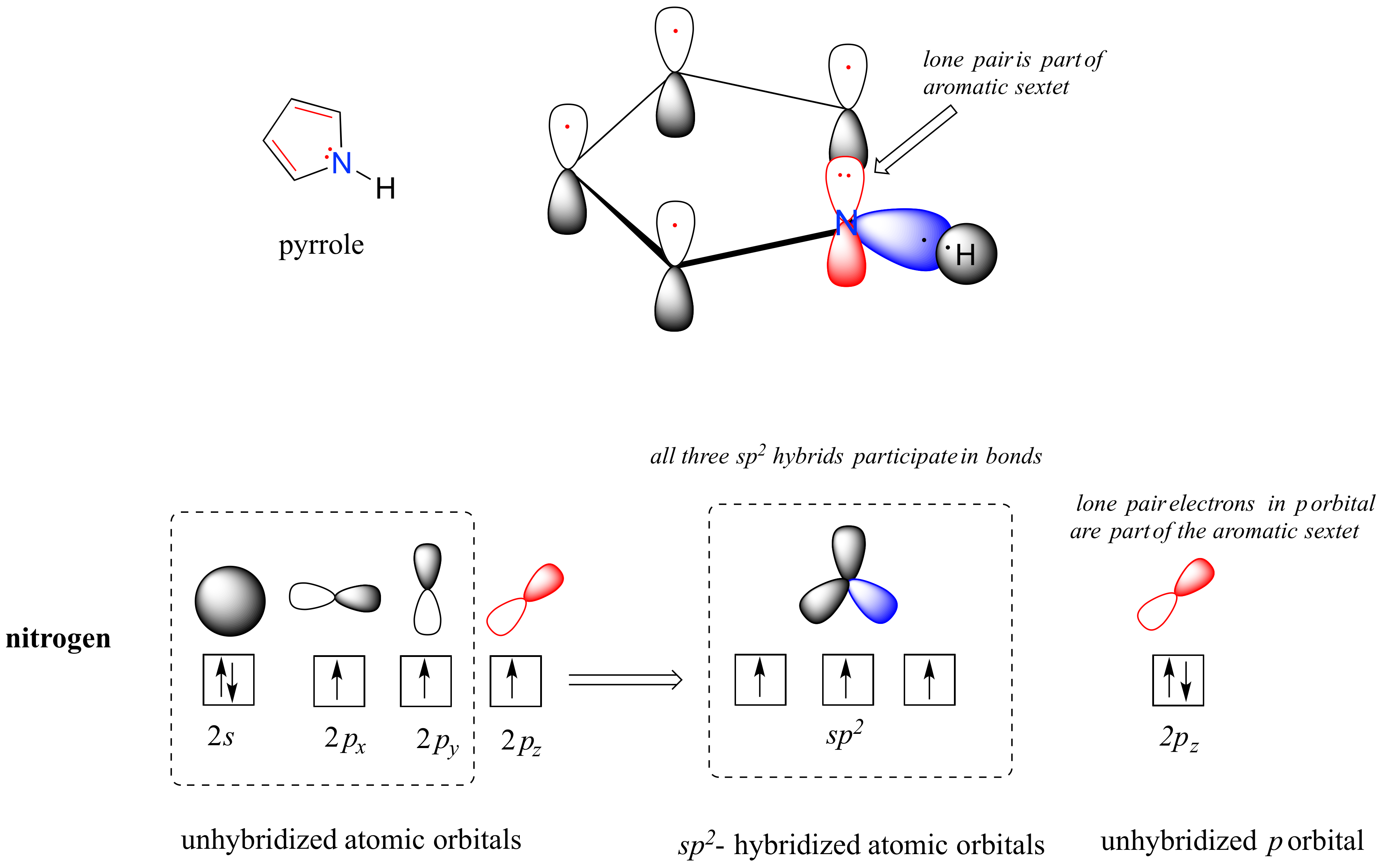
In a pyrrole ring, in contrast, the nitrogen lone pair is part of the aromatic sextet. This means that these electrons are very stable right where they are (in the aromatic system), and are much less available for bonding to a proton (and if they do pick up a proton, the aromic system is destroyed). For these reasons, pyrrole nitrogens are not strongly basic.
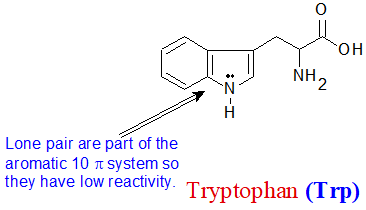
The aniline, pyridine, and pyrrole examples are good models for predicting the reactivity of nitrogen atoms in more complex ring systems (a huge diversity of which are found in nature). The tryptophan side chain, for example, contains a non-basic 'pyrrole-like' nitrogen, while adenine (a DNA/RNA base) contains all three types.
The lone pair electrons on the nitrogen of a nitrile are contained in a sp hybrid orbital. The 50% s character of an sp hybrid orbital means that the electrons are close to the nucleus and therefore not significantly basic.
Base Strength and pKa Values
Like ammonia, most amines are Brønsted and Lewis bases, but their base strength can be changed enormously by substituents. It is common to compare basicity's quantitatively by using the pKa's of their conjugate acids rather than their pKb's. Since pKa + pKb = 14, the higher the pKa the stronger the base, in contrast to the usual inverse relationship of pKa with acidity. Most simple alkyl amines have pKa's in the range 9.5 to 11.0, and their water solutions are basic (have a pH of 11 to 12, depending on concentration).
|
Compound |
 |
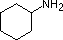 |
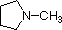 |
NH3 |
pyridine |
aniline |
4-nitroaniline |
pyrrole |
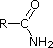 |
CH3C≡N acetonitrile |
|---|---|---|---|---|---|---|---|---|---|---|
| pKa | 11.0 | 10.7 | 10.7 | 9.3 | 5.2 | 4.6 | 1.0 | 0.0 | -1.0 | -10. |
The first four compounds in the table above are all weak bases. The last five compounds are significantly less basic to neutral and even acidic as a consequence of two possible factors:
- orbital hybridization
- electron delocalization through resonance.
In pyridine, the nitrogen is sp2 hybridized, and in nitriles (last entry) an sp hybrid nitrogen is part of the triple bond. In each of these compounds, the non-bonding electron pair is localized on the nitrogen atom, but increasing s-character brings it closer to the nitrogen nucleus, reducing its tendency to bond to a proton. For aniline and 4-nitroaniline, the nitrogen lone pair is stabilized through hyperconjugation with the aromatic ring. Pyrrole exhibits exceptional delocalization of the nitrogen electron pair because of its incorporation into the aromatic ring.
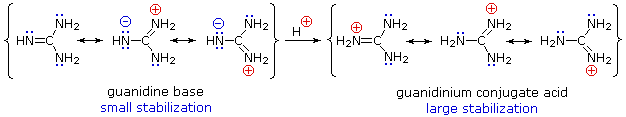
Although resonance delocalization generally reduces the basicity of amines, a dramatic example of the reverse effect is found in the compound guanidine (pKa = 13.6). Here, as shown below, resonance stabilization of the base is small, due to charge separation, while the conjugate acid is stabilized strongly by charge delocalization. Consequently, aqueous solutions of guanidine are nearly as basic as are solutions of sodium hydroxide.
Strong bases have weak conjugate acids, and weak bases have strong conjugate acids.
Amine Extraction in the Laboratory
Extraction is often employed in organic chemistry to purify compounds. Liquid-liquid extractions take advantage of the difference in solubility of a substance in two immiscible liquids (e.g. ether and water). The two immiscible liquids used in an extraction process are (1) the solvent in which the solids are dissolved, and (2) the extracting solvent. The two immiscible liquids are then easily separated using a separatory funnel. For amines one can take advantage of their basicity by forming the protonated salt (RNH2+Cl−), which is soluble in water. The salt will extract into the aqueous phase leaving behind neutral compounds in the non-aqueous phase. The aqueous layer is then treated with a base (NaOH) to regenerate the amine and NaCl. A second extraction-separation is then done to isolate the amine in the non-aqueous layer and leave behind NaCl in the aqueous layer.
Important Reagent Bases
The significance of all these acid-base relationships to practical organic chemistry lies in the need for organic bases of varying strength, as reagents tailored to the requirements of specific reactions. The common base sodium hydroxide is not soluble in many organic solvents, and is therefore not widely used as a reagent in organic reactions. Most base reagents are alkoxide salts, amines or amide salts. Since alcohols are much stronger acids than amines, their conjugate bases are weaker than amide bases, and fill the gap in base strength between amines and amide salts. In the following table, pKa again refers to the conjugate acid of the base drawn above it.
| Base Name | Pyridine | Triethyl Amine |
Hünig's Base | Barton's Base |
Potassium t-Butoxide |
Sodium HMDS | LDA |
|---|---|---|---|---|---|---|---|
| Formula |  |
(C2H5)3N | 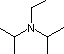 |
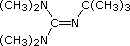 |
(CH3)3CO(–) K(+) | [(CH3)3Si]2N(–) Na(+) | [(CH3)2CH]2N(–) Li(+) |
| pKa | 5.3 | 10.7 | 11.4 | 14 | 19 | 26 | 35.7 |
Pyridine is commonly used as an acid scavenger in reactions that produce mineral acid co-products. Its basicity and nucleophilicity may be modified by steric hindrance, as in the case of 2,6-dimethylpyridine (pKa=6.7), or resonance stabilization, as in the case of 4-dimethylaminopyridine (pKa=9.7). Hünig's base is relatively non-nucleophilic (due to steric hindrance), and like DBU is often used as the base in E2 elimination reactions conducted in non-polar solvents. Barton's base is a strong, poorly-nucleophilic, neutral base that serves in cases where electrophilic substitution of DBU or other amine bases is a problem. The alkoxides are stronger bases that are often used in the corresponding alcohol as solvent, or for greater reactivity in DMSO. Finally, the two amide bases see widespread use in generating enolate bases from carbonyl compounds and other weak carbon acids.
Ammonium Salt Formation and Water Solubility
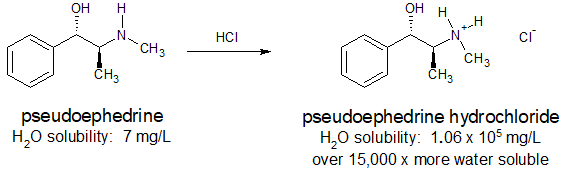
Many pharmaceuticals include amine functional groups. Sometimes the basicity of these amine groups is used to increase the water solubility of a drug so that it can be administered orally or intravenously. Ammonium salts are also thermally more stable and have less odor than their "free base" conjugates. The antihistamine, pseudoephedrine, reacts with hydrochloric acid to form the water soluble ammonium salt as shown below.
Exercise
4. Select the more basic compound from each of the following pairs of compounds.
(a)
(b)
(c)
5. The 4-methylbenzylammonium ion has a pKa of 9.51, and the butylammonium ion has a pKa of 10.59. Which is more basic? What's the pKb for each compound?
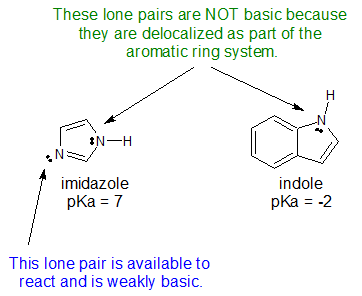
6. The structures for indole and imidazole are shown below. One compound has a pKa of -2 while the other compound has a pKa of 7. Assign the pKa values to the corresponding compound and explain your reasoning.
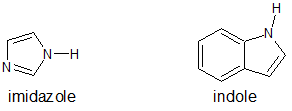
- Answer
-
4.
(a)
(b)
(c)
5. The butylammonium is more basic. The pKb for butylammonium is 3.41, the pKb for 4-methylbenzylammonium is 4.49.
6.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)