6.3: Reaction Mechanisms Involving Polar Functional Groups: Using "Electron-Pushing'" Arrows
- Page ID
- 32368
After completing this section, you should be able to
- explain the difference between heterolytic and homolytic bond breakage, and between heterogenic and homogenic bond formation.
- state the two reaction types involved in symmetrical and unsymmetrical processes.
Make certain that you can define, and use in context, the key terms below.
- heterogenic
- heterolytic
- homogenic
- homolytic
- polar reaction
- radical reaction
- reaction mechanism
Upon first reading first four key terms, it is easy to be puzzled. The ending of the word tells you whether a bond is being formed (‑genic) or broken (‑lytic), while the root of the word describes the nature of that formation or decomposition. So hetero (meaning different) reactions involve asymmetrical bond making (or breaking) and homo (meaning same) involve symmetrical processes.
Because one pair of electrons constitutes a single bond, the unsymmetrical making or breaking of that bond in a hetero processes are described as polar reactions. Similarly, symmetrical homo processes of bond making and breaking are called radical reactions. Radicals (sometimes referred to as free radicals) are highly reactive neutral chemical species with one unpaired electron. In later sections we discuss radical and polar reactions in more detail.
The Arrow Notation in Mechanisms
Since chemical reactions involve the breaking and making of bonds, a consideration of the movement of bonding (and non-bonding) valence shell electrons is essential to this understanding. It is now common practice to show the movement of electrons with curved arrows, and a sequence of equations depicting the consequences of such electron shifts is termed a mechanism. In general, two kinds of curved arrows are used in drawing mechanisms:
| A full head on the arrow indicates the movement or shift of an electron pair: | ||
| A partial head (fishhook) on the arrow indicates the shift of a single electron: |
The use of these symbols in bond-breaking and bond-making reactions is illustrated below. If a covalent single bond is broken so that one electron of the shared pair remains with each fragment, as in the first example, this bond-breaking is called homolysis. If the bond breaks with both electrons of the shared pair remaining with one fragment, as in the second and third examples, this is called heterolysis.
| Bond-Breaking | Bond-Making |
Other Arrow Symbols
Chemists also use arrow symbols for other purposes, and it is essential to use them correctly.
| The Reaction Arrow | The Equilibrium Arrow | The Resonance Arrow |
The following equations illustrate the proper use of these symbols:
Reactive Intermediates
The products of bond breaking, shown above, are not stable in the usual sense, and cannot be isolated for prolonged study. Such species are referred to as reactive intermediates, and are believed to be transient intermediates in many reactions. The general structures and names of four such intermediates are given below.
| Charged Intermediates | Uncharged Intermediates |
|---|---|
a carbocation |
a radical |
a carbanion |
a carbene |
A pair of widely used terms, related to the Lewis acid-base notation, should also be introduced here.
- Electrophile: An electron deficient atom, ion or molecule that has an affinity for an electron pair, and will bond to a base or nucleophile.
- Nucleophile: An atom, ion or molecule that has an electron pair that may be donated in bonding to an electrophile (or Lewis acid).
Using these definitions, it is clear that carbocations ( called carbonium ions in the older literature ) are electrophiles and carbanions are nucleophiles. Carbenes have only a valence shell sextet of electrons and are therefore electron deficient. In this sense they are electrophiles, but the non-bonding electron pair also gives carbenes nucleophilic character. As a rule, the electrophilic character dominates carbene reactivity. Carbon radicals have only seven valence electrons, and may be considered electron deficient; however, they do not in general bond to nucleophilic electron pairs, so their chemistry exhibits unique differences from that of conventional electrophiles. Radical intermediates are often called free radicals.
The importance of electrophile / nucleophile terminology comes from the fact that many organic reactions involve at some stage the bonding of a nucleophile to an electrophile, a process that generally leads to a stable intermediate or product. Reactions of this kind are sometimes called ionic reactions, since ionic reactants or products are often involved. Some common examples of ionic reactions and their mechanisms may be examined below.
The shapes ideally assumed by these intermediates becomes important when considering the stereochemistry of reactions in which they play a role. A simple tetravalent compound like methane, CH4, has a tetrahedral configuration. Carbocations have only three bonds to the charge bearing carbon, so it adopts a planar trigonal configuration. Carbanions are pyramidal in shape ( tetrahedral if the electron pair is viewed as a substituent), but these species invert rapidly at room temperature, passing through a higher energy planar form in which the electron pair occupies a p-orbital. Radicals are intermediate in configuration, the energy difference between pyramidal and planar forms being very small. Since three points determine a plane, the shape of carbenes must be planar; however, the valence electron distribution varies.
Ionic Reactions
The principles and terms introduced in the previous sections can now be summarized and illustrated by the following three examples. Reactions such as these are called ionic or polar reactions, because they often involve charged species and the bonding together of electrophiles and nucleophiles. Ionic reactions normally take place in liquid solutions, where solvent molecules assist the formation of charged intermediates.
Substitution Reaction
The substitution reaction shown below can be viewed as taking place in three steps. The first is an acid-base equilibrium, in which HCl protonates the oxygen atom of the alcohol. The resulting conjugate acid then loses water in a second step to give a carbocation intermediate. Finally, this electrophile combines with the chloride anion nucleophile to give the final product.
Addition Reaction
The addition reaction shown below can be viewed as taking place in two steps. The first step can again be considered an acid-base equilibrium, with the pi-electrons of the carbon-carbon double bond functioning as a base. The resulting conjugate acid is a carbocation, and this electrophile combines with the nucleophilic bromide anion.
Elemination Reaction
The elimination reaction shown below takes place in one step. The bond breaking and making operations that take place in this step are described by the curved arrows. The initial stage may also be viewed as an acid-base interaction, with hydroxide ion serving as the base and a hydrogen atom component of the alkyl chloride as an acid. rearrangement (tautomerism)
Tautomerization Reaction
There are many kinds of molecular rearrangements. The examples shown below are from an important class called tautomerization or, more specifically, keto-enol tautomerization. Tautomers are rapidly interconverted constitutional isomers, usually distinguished by a different bonding location for a labile hydrogen atom (colored red here) and a differently located double bond. The equilibrium between tautomers is not only rapid under normal conditions, but it often strongly favors one of the isomers (acetone, for example, is 99.999% keto tautomer). Even in such one-sided equilibria, evidence for the presence of the minor tautomer comes from the chemical behavior of the compound. Tautomeric equilibria are catalyzed by traces of acids or bases that are generally present in most chemical samples.
The Brønsted-Lowry definition of acidity
We’ll begin our discussion of acid-base chemistry with a couple of essential definitions. The first of these definitions was proposed in 1923 by the Danish chemist Johannes Brønsted and the English chemist Thomas Lowry, and has come to be known as the Brønsted-Lowry definition of acids and bases. An acid, by the Brønsted-Lowry definition, is a species which is able to donate a proton (H+), while a base is a proton acceptor. We have already discussed in the previous chapter one of the most familiar examples of a Brønsted-Lowry acid-base reaction, between hydrochloric acid and hydroxide ion:

In this reaction, a proton is transferred from HCl (the acid, or proton donor) to hydroxide (the base, or proton acceptor). As we learned in the previous chapter, curved arrows depict the movement of electrons in this bond-breaking and bond-forming process.
After a Brønsted-Lowry acid donates a proton, what remains – in this case, a chloride ion – is called the conjugate base. Chloride is thus the conjugate base of hydrochloric acid. Conversely, when a Brønsted-Lowry base accepts a proton it is converted into its conjugate acid form: water is thus the conjugate acid of hydroxide.
We can also talk about conjugate acid/base pairs: the two acid/base pairs involved in our first reaction are hydrochloric acid/chloride and hydroxide/water. In this next acid-base reaction, the two pairs involved are acetate/acetic acid and methyl ammonium/methylamine:
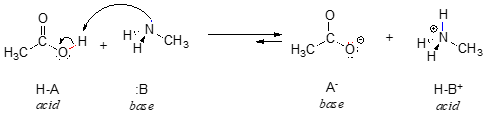
Throughout this text, we will often use the abbreviations HA and :B in order to refer in a general way to acidic and basic reactants:
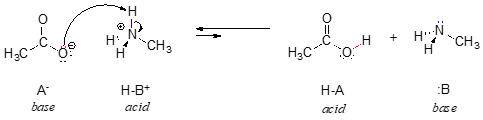
In order to act as a proton acceptor, a base must have a reactive pair of electrons. In all of the examples we shall see in this chapter, this pair of electrons is a non-bonding lone pair, usually (but not always) on an oxygen, nitrogen, sulfur, or halogen atom. When acetate acts as a base in the reaction shown above, for example, one of its oxygen lone pairs is used to form a new bond to a proton. The same can be said for an amine acting as a base. Clearly, methyl ammonium ion cannot act as a base – it does not have a reactive pair of electrons with which to accept a new bond to a proton.
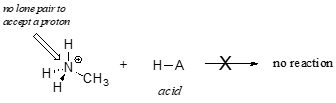
Later, in chapter 15, we will see several examples where the (relatively) reactive pair of electrons in a \( \pi \) bond act in a basic fashion.
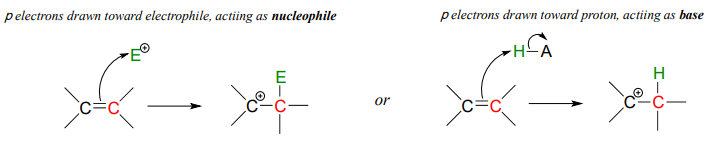
In this chapter, we will concentrate on those bases with non-bonding (lone pair) electrons.
| Example |
Exercise 7.1: Draw structures for the missing conjugate acids or conjugate bases in the reactions below.
Contributors
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

