17.5: Amino Acids
- Page ID
- 30813
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Common amino acids
There are 20 common amino acids. They are composed of C, H, O, N and S atoms. They are structurally and chemically different, and also differ in size and volume. Some are branched structures, some are linear, some have ring structures. One of the 20 common amino acids is actually an imino acid. A typical grouping of their chemical nature is as follows:
- Nonpolar (hydrocarbons and one sulfur-containing amino acid). Dispersion forces and hydrophobic effects predominate in their interactions. They cannot H-bond with water and these side chains have a characteristic hydrophobic effect in water.
- Polar uncharged. Contain functional groups that can H-bond with water and other amino acids. Include C, H, O, N and S atoms.
- Acidic. Contain a carboxylic acid functional group with a negative charge at neutral pH. Can H-bond with water, can form ionic interactions, and can also serve as nucleophiles or participate in acid-base chemistry.
- Basic. Nitrogen containing bases (e.g. guanidino, imidazole or amino groups) with a net positive charge at neutral pH. Can serve as proton donors in chemical reactions, and form ionic interactions.
The amino acids have a name, as well as a three letter or single letter mnemonic code:
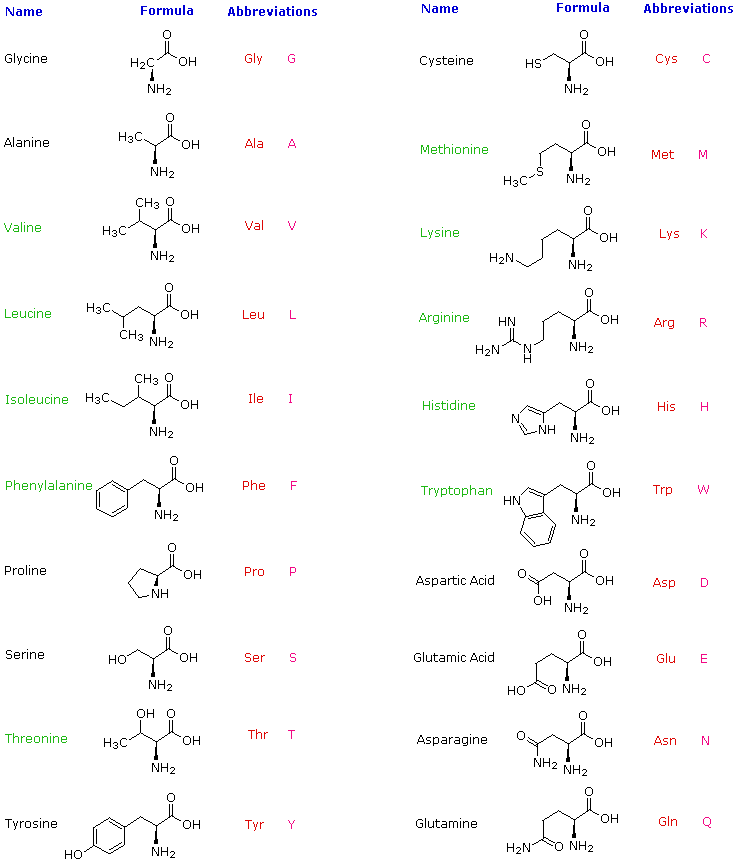
Strereochemistry in amino acids
With the exception of glycine, all the 19 other common amino acids have a uniquely different functional group on the central tetrahedral alpha carbon (i.e. Ca )
- The C a is termed "chiral" to indicate there are four different constituents and that the Ca is asymmetric
- Since the C a is asymmetric there exists two possible, non-superimposable, mirror images of the amino acids:
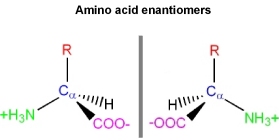
- How are these two uniquely different structures distinguished?
The D, L system
Glyceraldehyde contains a chiral carbon, and therefore, there are two enantiomers of this molecule.
- One is labeled the "L" form, and the other the "D" form
- This is the frame of reference used to describe amino acid enantiomers as being either the "L" or "D" form
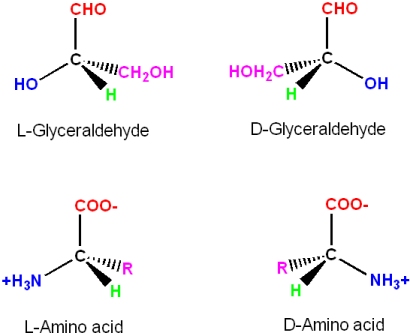
Even though the two enantiomers would seem to be essentially equivalent to each other, all common amino acids are found in the "L" enantiomer in living systems
- When looking down the H-C
- a bond towards the Ca there is a mnemonic to identify the L-enantiomer of amino acids (note: in this view the three functional groups are pointing away from you, and not towards you; the H atom is omitted for clarity - but it would be in front of the C)
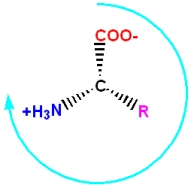
- Starting with the carbonyl functional group, and going clockwise around the C
- a of the L-enantiomer, the three functional groups spell out the word CORN.
- If you follow the above instructions, it will spell out CONR (a silly, meaningless word) for the D-enantiomer
The R,S system of naming chiral centers
- A relative ranking of the "priority" of various functional groups is given as:
SH > OH > NH2 > COOH > CHO > CH2OH > CH3 > H
- A chiral center has four different functional groups. Identify the functional group with the lowest priority
- View the chiral center down the bond from the chiral center to the lowest priority atom
- don't confuse this with the CORN mnemonic method of identifying the L-amino acid chirality by viewing from the H to the Ca )
- Assign priorities to the three other functional groups connected to the chiral center, using the above ranking
- If the priorities of these other groups goes in a clockwise rotation, the chirality is "R". If the priorities of these other groups goes counterclockwise, the chirality is "S". (Note that this assignment has nothing to do with optical activity, and is not using L-glyceraldehyde as a reference molecule)

Spectroscopic properties of amino acids
This refers to the ability of amino acids to absorb or emit electromagnetic energy at different wavelengths (i.e. energies)
- No amino acids absorb light in the visible spectrum (i.e. they are "colorless").
- If proteins have color (e.g. hemoglobin is red) it is because they contain a bound, non-protein atom, ion or molecule; iron in this case)
- All amino acids absorb in the infrared region (longer wavelengths, weaker energy than visible light)
- Some amino acids absorb in the ultraviolet spectrum (shorter wavelengths, higher energy than visible light)
- Absorption occurs as electrons rise to higher energy states
- Electrons in aromatic ring structures absorb in the u.v. spectrum. Such structures comprise the side chains of
- tryptophan, tyrosine and phenylalanine.
Amino acids as zwitterions
An amino acid has both a basic amine group and an acidic carboxylic acid group.
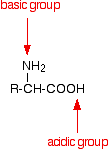
There is an internal transfer of a hydrogen ion from the -COOH group to the -NH2 group to leave an ion with both a negative charge and a positive charge. This is called a zwitterion.

This is the form that amino acids exist in even in the solid state. If you dissolve the amino acid in water, a simple solution also contains this ion. A zwitterion is a compound with no overall electrical charge, but which contains separate parts which are positively and negatively charged.
Adding an alkali to an amino acid solution
If you increase the pH of a solution of an amino acid by adding hydroxide ions, the hydrogen ion is removed from the -NH3+ group.
![]()

You could show that the amino acid now existed as a negative ion using electrophoresis. In its simplest form, electrophoresis can just consist of a piece of moistened filter paper on a microscope slide with a crocodile clip at each end attached to a battery. A drop of amino acid solution is placed in the center of the paper.
Although the amino acid solution is colourless, its position after a time can be found by spraying it with a solution of ninhydrin. If the paper is allowed to dry and then heated gently, the amino acid shows up as a coloured spot. The amino acid would be found to travel towards the anode (the positive electrode).
Adding an acid to an amino acid solution
If you decrease the pH by adding an acid to a solution of an amino acid, the -COO- part of the zwitterion picks up a hydrogen ion.
![]()
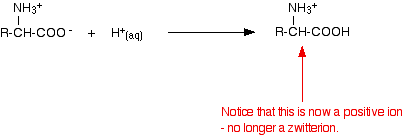
This time, during electrophoresis, the amino acid would move towards the cathode (the negative electrode).
Shifting the pH from one extreme to the other
Suppose you start with the ion we've just produced under acidic conditions and slowly add alkali to it. That ion contains two acidic hydrogens - the one in the -COOH group and the one in the -NH3+ group. The more acidic of these is the one in the -COOH group, and so that is removed first - and you get back to the zwitterion.
![]()
![]()
So when you have added just the right amount of alkali, the amino acid no longer has a net positive or negative charge. That means that it wouldn't move towards either the cathode or anode during electrophoresis. The pH at which this lack of movement during electrophoresis happens is known as the isoelectric point of the amino acid. This pH varies from amino acid to amino acid. If you go on adding hydroxide ions, you will get the reaction we've already seen, in which a hydrogen ion is removed from the -NH3+ group.
![]()
![]()
You can, of course, reverse the whole process by adding an acid to the ion we've just finished up with. That ion contains two basic groups - the -NH2 group and the -COO- group. The -NH2 group is the stronger base, and so picks up hydrogen ions first. That leads you back to the zwitterion again.
![]()
![]()
. . . and, of course, you can keep going by then adding a hydrogen ion to the -COO- group.
![]()
![]()
The Isoelectric Point
As defined above, the isoelectric point, pI, is the pH of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. In other words, the positively charged groups are exactly balanced by the negatively charged groups. For simple amino acids such as alanine, the pI is an average of the pKa's of the carboxyl (2.34) and ammonium (9.69) groups. Thus, the pI for alanine is calculated to be: (2.34 + 9.69)/2 = 6.02, the experimentally determined value. If additional acidic or basic groups are present as side-chain functions, the pI is the average of the pKa's of the two most similar acids. To assist in determining similarity we define two classes of acids. The first consists of acids that are neutral in their protonated form (e.g. CO2H & SH). The second includes acids that are positively charged in their protonated state (e.g. -NH3+). In the case of aspartic acid, the similar acids are the alpha-carboxyl function (pKa = 2.1) and the side-chain carboxyl function (pKa = 3.9), so pI = (2.1 + 3.9)/2 = 3.0. For arginine, the similar acids are the guanidinium species on the side-chain (pKa = 12.5) and the alpha-ammonium function (pKa = 9.0), so the calculated pI = (12.5 + 9.0)/2 = 10.75.
Why isn't the isoelectric point of an amino acid at pH 7?
When an amino acid dissolves in water, the situation is a little bit more complicated than we tend to pretend at this level. The zwitterion interacts with water molecules - acting as both an acid and a base. As an acid:
![]()
![]()
The -NH3+ group is a weak acid and donates a hydrogen ion to a water molecule. Because it is only a weak acid, the position of equilibrium will lie to the left.
As a base:
![]()
![]()
The -COO- group is a weak base and takes a hydrogen ion from a water molecule. Again, the equilibrium lies to the left.
When you dissolve an amino acid in water, both of these reactions are happening. However, the positions of the two equilibria aren't identical - they vary depending on the influence of the "R" group. In practice, for the simple amino acids we have been talking about, the position of the first equilibrium lies a bit further to the right than the second one. That means that there will be rather more of the negative ion from the amino acid in the solution than the positive one.
In those circumstances, if you carried out electrophoresis on the unmodified solution, there would be a slight drift of amino acid towards the positive electrode (the anode). To stop that, you need to cut down the amount of the negative ion so that the concentrations of the two ions are identical. You can do that by adding a very small amount of acid to the solution, moving the position of the first equilibrium further to the left. Typically, the pH has to be lowered to about 6 to achieve this. For glycine, for example, the isoelectric point is pH 6.07; for alanine, 6.11; and for serine, 5.68.
Contributors
Jim Clark (Chemguide.co.uk)
Charles Ophardt (Professor Emeritus, Elmhurst College); Virtual Chembook
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

