4.2: Cycloalkanes
- Page ID
- 30339
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Cycloalkanes are cyclic hydrocarbons, meaning that the carbons of the molecule are arranged in the form of a ring. Cycloalkanes are also saturated, meaning that all of the carbons atoms that make up the ring are single bonded to other atoms (no double or triple bonds). There are also polycyclic alkanes, which are molecules that contain two or more cycloalkanes that are joined, forming multiple rings.
Introduction
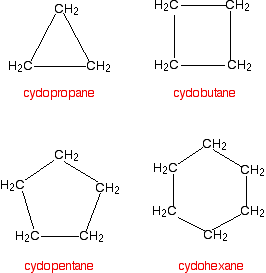
Many organic compounds found in nature or created in a laboratory contain rings of carbon atoms with distinguishing chemical properties; these compounds are known as cycloalkanes. Cycloalkanes only contain carbon-hydrogen bonds and carbon-carbon single bonds, but in cycloalkanes, the carbon atoms are joined in a ring. The smallest cycloalkane is cyclopropane.
If you count the carbons and hydrogens, you will see that they no longer fit the general formula \(C_nH_{2n+2}\). By joining the carbon atoms in a ring,two hydrogen atoms have been lost. The general formula for a cycloalkane is \(C_nH_{2n}\). Cyclic compounds are not all flat molecules. All of the cycloalkanes, from cyclopentane upwards, exist as "puckered rings". Cyclohexane, for example, has a ring structure that looks like this:
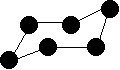
Figure 2: This is known as the "chair" form of cyclohexane from its shape, which vaguely resembles a chair. Note: The cyclohexane molecule is constantly changing, with the atom on the left, which is currently pointing down, flipping up, and the atom on the right flipping down. During this process, another (slightly less stable) form of cyclohexane is formed known as the "boat" form. In this arrangement, both of these atoms are either pointing up or down at the same time
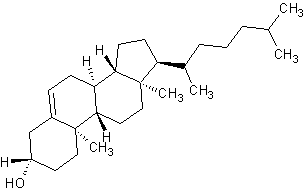
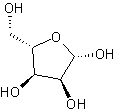
In addition to being saturated cyclic hydrocarbons, cycloalkanes may have multiple substituents or functional groups that further determine their unique chemical properties. The most common and useful cycloalkanes in organic chemistry are cyclopentane and cyclohexane, although other cycloalkanes varying in the number of carbons can be synthesized. Understanding cycloalkanes and their properties are crucial in that many of the biological processes that occur in most living things have cycloalkane-like structures.
 |  |
|
| Glucose (6 carbon sugar) | Ribose (5 carbon sugar) | Cholesterol (polycyclic) |
Although polycyclic compounds are important, they are highly complex and typically have common names accepted by IUPAC. However, the common names do not generally follow the basic IUPAC nomenclature rules. The general formula of the cycloalkanes is \(C_nH_{2n}\) where \(n\) is the number of carbons. The naming of cycloalkanes follows a simple set of rules that are built upon the same basic steps in naming alkanes. Cyclic hydrocarbons have the prefix "cyclo-".
Contents
For simplicity, cycloalkane molecules can be drawn in the form of skeletal structures in which each intersection between two lines is assumed to have a carbon atom with its corresponding number of hydrogens.
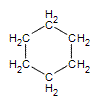.gif?revision=1&size=bestfit&width=85&height=92) same as
same as 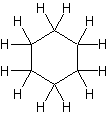 same as
same as 
| Cycloalkane | Molecular Formula | Basic Structure |
| Cyclopropane | C3H6 |  |
| Cyclobutane | C4H8 |  |
| Cyclopentane | C5H10 |  |
| Cyclohexane | C6H12 |  |
| Cycloheptane | C7H14 | 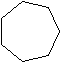 |
| Cyclooctane | C8H16 | 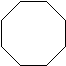 |
| Cyclononane | C9H18 | 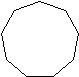 |
| Cyclodecane | C10H20 | 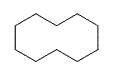 |
Contributors
Jim Clark (Chemguide.co.uk)
- Pwint Zin