11.6: Alkene Metathesis
- Page ID
- 13912
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Olefin metathesis, or alkene metathesis, is an important process in petroleum refining and in the synthesis of important compounds such as pharmaceuticals. The mechanism of olefin metathesis is related to pericyclic reactions like Diels Alder and [2+2] reactions. In other words, it occurs through the concerted interaction of one molecule with another.
In petroleum refining, heating alkenes over metal oxide surfaces results in the formation of longer-chain alkenes. In particular, terminal olefins (with the double bond at the end of the chain) are converted into internal olefins (with the double bond somewhere in the middle of the chain).
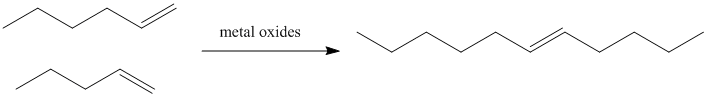
Figure PR4.1. Olefin metathesis produces longer-chain "internal olefins" from shorter chain "terminal olefins".
What does that reaction have to do with addition reactions involving double bonds?
Clearly, the alkenes have double bonds. In addition, so do the metal oxides. Metal atoms inside the metal oxides are bridged together by oxygen atoms. The surface of the metal oxides may be covered with a mixture of hydroxyl groups as well as terminal oxides (M=O groups). The terminal oxides on the surface are the important part of the catalyst.
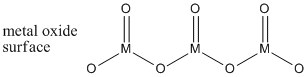
Figure PR4.2. A simplified structure of a chunk of metal oxide surface.
When metal alkylidene complexes were developed in the 1970's, it was found that they, too, could catalyze this reaction.
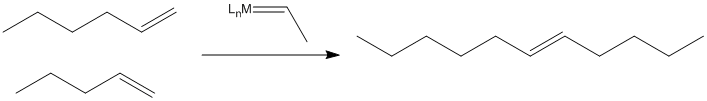
Figure PR4.3. Olefin metathesis produces longer-chain "internal olefins" from shorter chain "terminal olefins"
In fact, scientists working in petroleum chemistry soon came to believe that metal oxides on the catalyst surface were converted to alkylidenes, which then carried out the work of olefin metathesis. The reaction, it turns out, involves a [2+2] cycloaddition of an alkene to a metal alkylidene (to the metal-carbon double bond). This reaction results in a four-membered ring, called a metallacyclobutane. The [2+2] cycloaddition is quickly followed by the reverse reaction, a retro-[2+2]. The metallacyclobutane pops open to form two new double bonds.
The Chauvin Mechanism
This mechanism is called the Chauvin mechanism, after its first proponent, Yves Chauvin of the French Petroleum Institute. Chauvin's proposal of this mechanism shortly after the discovery of metal alkylidenes by Dick Schrock at DuPont earned him a Nobel Prize in 2005. Chauvin and Schrock shared the prize with Bob Grubbs, who made it possible for the reaction to be adapted easily to the synthesis of complex molecules such as pharmaceuticals.
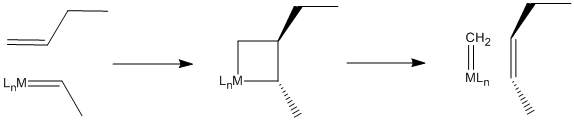
Figure PR4.4. The Chauvin mechanism for olefin metathesis.
Why does olefin metathesis lead to the formation of internal alkenes? The [2+2] addition and retro-[2+2] reactions occur in equilibrium with each other. Each time the metallacyclobutane forms, it can form two different pairs of double bonds through the retro reaction. In the presence of terminal alkenes, one of those pairs of alkenes will eventually include ethene. Since ethene is a gas, it is easily lost from the system, and equilibrium shifts to the right in the equation below.
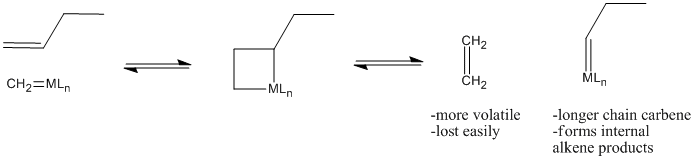
Figure PR4.5. Olefin metathesis produces longer-chain "internal olefins" from shorter chain "terminal olefins".
That leaves a longer-chain alkylidene on the metal, ready to be attached to another long chain through subsequent [2+2] addition and reversion reactions.
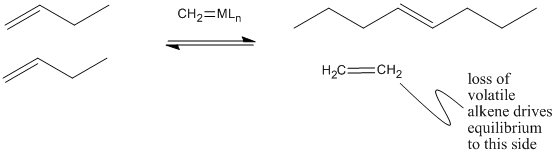
Figure PR4.6. Olefin metathesis produces longer-chain "internal olefins" from shorter chain "terminal olefins".
In most cases, a [2+2] addition will not work unless photochemistry is involved, but it does work with metal alkylidenes. The reason for this exception is thought to involve the nature of the metal-carbon double bond. In contrast to an orbital picture for an alkene, an orbital picture for an alkylidene pi bond suggests orbital symmetry that can easily interact with the LUMO on an alkene. That's because a metal-carbon \(pi\) bond likely involves a d orbital on the metal, and the d orbital has lobes alternating in phase like a \(pi\) antibonding orbital.
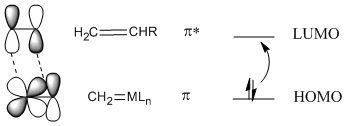
Figure PR4.7. Olefin metathesis produces longer-chain "internal olefins" from shorter chain "terminal olefins".

