11: Strategies in Steroids Synthesis
- Page ID
- 21816
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The main skeletal features in steroid rings are depicted by the two figures shown in Figure 11.1. The same numbering system is maintained even while describing part structures obtained as synthetic intermediates.
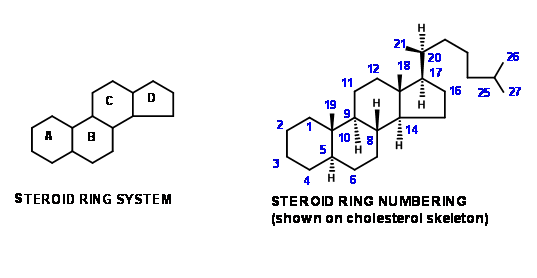
This convention helps us to follow the development of structural features through long synthetic schemes. Students should also become familiar with another convention followed by chemists to categorize synthetic schemes, originally evolved for steroids. Similar descriptions are also found in alkaloid chemistry. ‘An \(AB \rightarrow ABC \rightarrow ABCD\) Approach’ would mean that a naphthalene skeleton (either aromatic or suitable perhydro- skeleton) is chosen as SM. The C ring is then constructed on the AB rings. The D ring is then formed by ring closure. An example to this strategy is Bechmann’s synthesis (1940). Such descriptions do not indicate any details like the substitution patterns on the SM or synthetic intermediates nor do they spill light on stereochemical details. These details are discussed in the synthetic schemes.
Bechmann’s Synthesis (1940) of Equilenin
This classical synthesis exploits the chemistry of the naphthalene ring. 1-naphthylamine-2-sulphonic acid was chosen as the AB ring (Fig 11.2). The sulphonic
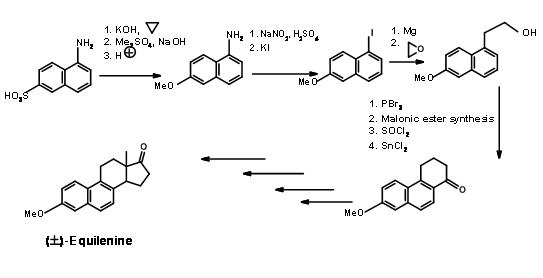
acid moiety was converted to a phenol and protected as the methyl ether. The amine moiety was converted to iodide through diazotisation. The C and D rings were then built on the AB rings
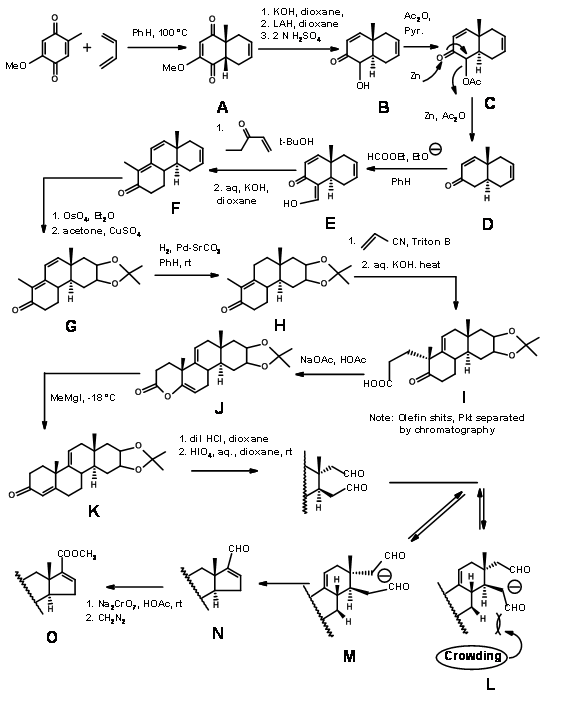
Woodward’s synthesis of Cholesterol:
Woodward’s synthesis of Cholesterol (J. Am. Chem. Soc., 73, 2403, 3547, 3548 (1951); ibid, 74, 4223 (1952) (Fig 11.3) could be described as \(C \rightarrow CD \rightarrow BCD \rightarrow ABCD\) Approach. Since the D ring remains D-homo until the last step of ring construction and the required 5-membered ring was obtained only after a ring contraction, it could also be termed as \(C \rightarrow BC \rightarrow ABC \rightarrow ABCD\) Approach. Remember that this molecule has 8 asymmetric centers and could therefore have 28 (256) optical isomers. This synthesis envisaged the synthesis of just one set of diastereomers. This stereospecific synthesis incorporated all the stereopoints in a stereo- and regiospecific manner. The D ring provides an anchor for variations in the chain. The double bond on the C ring allowed an opening for the synthesis of cortisone. The legends on the arrows show the reactions.

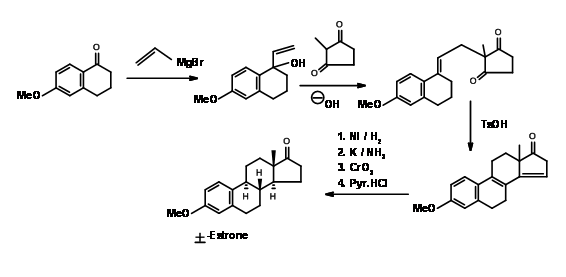
Synthesis of Estrone
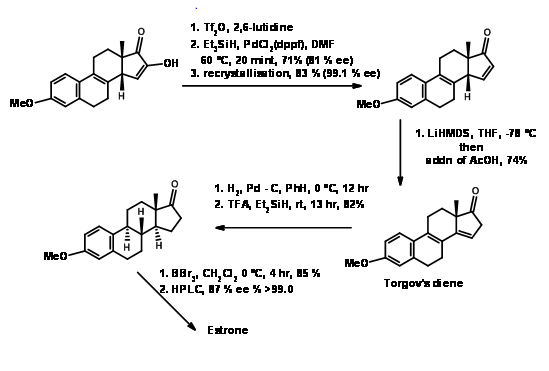
Estrone has attracted several chemists as a target for executing new methodologies on this complex yet useful steroid. A very popular method for the introduction of the D ring, followed by cyclization of the C ring in steroid synthesis was introduced by Torgov (1950, 63). His synthesis of Estrone is shown in Figure 11.4. His procedure for \(AB \rightarrow ABD \rightarrow ABCD\) Approach became very popular. Several modifications were later developed to further improve this methodology.
Bartlet used a mechanistic transform in his synthesis of Estrone. He applied a biogenetic-type cyclisation for his \(\ce{A -> AD -> ABCD}\) approach shown in Figure 11.5. A furan ring served as a masked 1,4-diketone needed for the D ring. Also note the rearrangement transform used for the stereospecific introduction of the angular methyl group at C13 position in the last step of the synthesis.
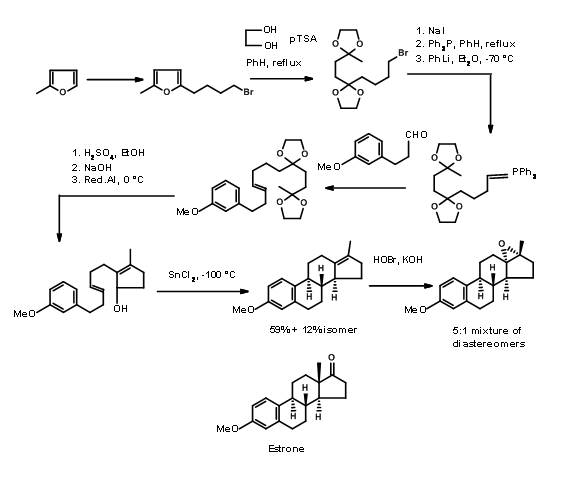
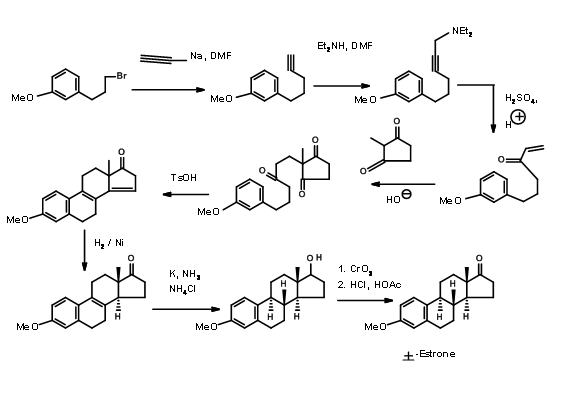
Hughes (1960) relied on aldol transforms for his A AD ABCD Approach. Here he made use of the reliable reduction protocols developed by several workers for controlling the stereochemistry at ring junctions (Fig 11.6)
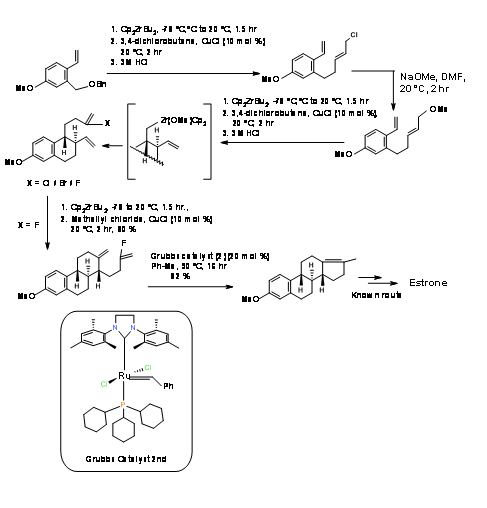
P. Hermann et.al., (J. Org. Chem., 73, 6202 (2008)) had attempted a sequential Media:Ring Closure Reactions (RCR) strategy for the stereospecific synthesis of Estrone. These workers relied on the CP2ZrBU2 catalyst studied extensively in their laboratory and developed a short 9-step synthesis from known diene (A AB ABC ABCD Approach)(Fig 11.7). Cyclisation of the B ring proceeded satisfactorily. Cylisation of the C ring was sensitive to the halogen. After several attempt, cyclisation proceeded well with the vinyl fluoride. The formation of the D ring using Zr catalyst was unsuccessful. The ring closure of the D ring was finally accomplished using the second-generation Media:Grubbs catalyst. The final modification of the D-ring has been already reported by Bartlett P.A et.al., ( J. Am. Chem. Soc., 95, 7501 (1973) thereby completing a formal synthesis of Estrone.
Marko Weimar et.al., (J. Org. Chem., 75, 2718 (2010)) have reported a very successful DA Transform for the formation of CD ring from Dane’s diene as AB ring and a D ring as dienoplile (Fig 11.8) (AB ABCD Approach; see references cited for similar approaches).
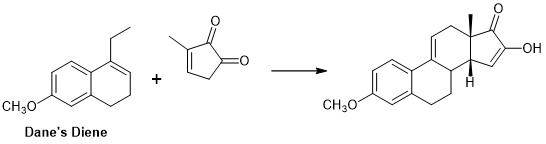
Figure 11.8
Key for the success of this strategy was the metal-free chiral catalyst, which they successfully tailored by the introduction of three H-bonding sites. The catalyst that worked efficiently was the Media:axially chiral amidine salt 11.9.
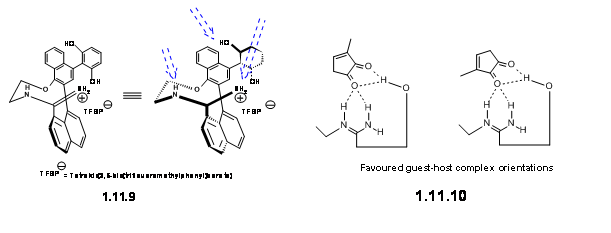
They have suggested that this ligand formed a Host-Guest complex with the dienoplile as depicted in 11.10.
Under these conditions, the Diels-Alder reaction proceeded in excellent yield. This compound was converted to Estrone as shown in Figure 11.11
Synthesis of Cortisone
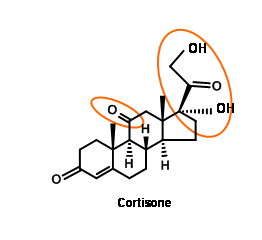
Cortisone attracted the attention of several synthetic chemists, because this wonder drug was available only in minute quantities from animal sources. Two challenging features in the structure of cortisone were the keto- group at C11 and the 1,2,3- oxygenation pattern at the two-carbon side chain at C17.
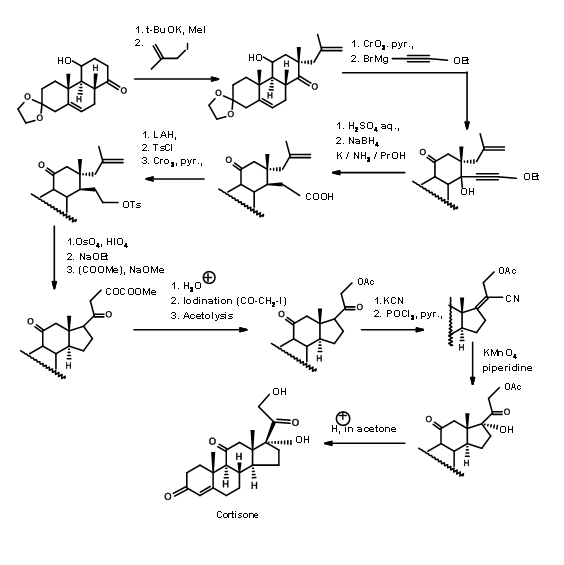
Sarrett (J. Am. Chem. Soc., 74, 4974 (1952); J. Am. Chem. Soc., 76, 5031 (1954)) used an ABC ABCD Approach. His starting molecule had all the features needed for the A, B and C rings of Cortisone. It had a C11 –OH group and a conveniently placed ketone for building the D ring. They exploited the known stereoelectronic constraints of anion reactions on a rigid 6-membered ring to construct the C/D trans ring junction (Fig 11.12). This cyclisation has two noteworthy features. The olefin served as a masked ketone. Unlike Woodward’s Cholesterol synthesis (Fig 11.3), which had a competing site for anion formation, in this scheme only one anion is feasible leading to predictable product.
An interesting new approach in steroid synthesis came from Y. Horiguchi (J. Org. Chem., 51, 4325 (1986)). This could be described as CD BCD ABCD Approach. Note that the C11 oxygenation and the carbons needed for the A ring came through a single oxidation step, as envisaged in their retroanalysis shown in Figure 11.13.
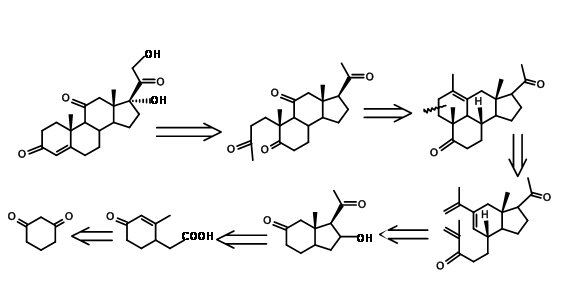
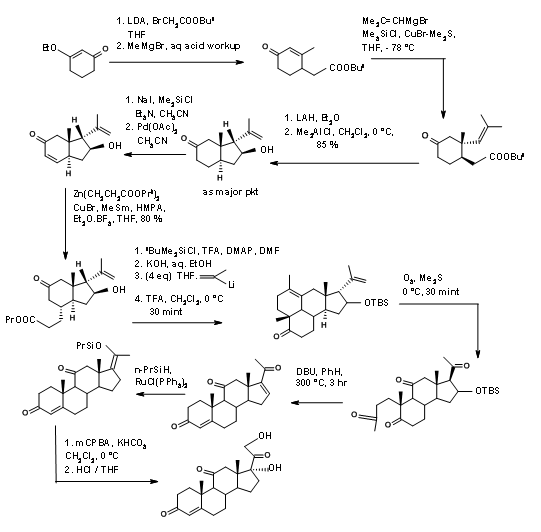
A detailed synthetic scheme of Horiguchi is shown in Figure 11.14. The strategy for formation of A as well as the D ring is of interest in this synthesis.
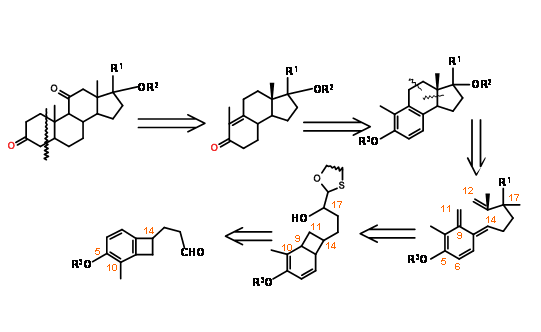
Nemoto’s retroanalysis (J. Org. Chem., 55, 5625 (1990)) provided an interesting electrocyclic ring opening – cycloaddition strategy for a B BCD ABCD Approach (Fig 11.15).
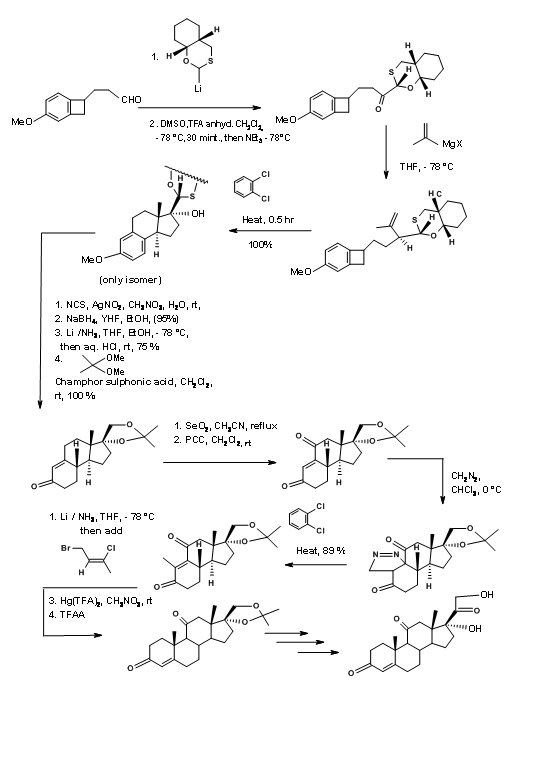
The synthetic scheme is shown in Figure 11.16. Note the versatile chiral auxiliary in the first step that serves several useful bond-forming purposes in addition to guiding three asymmetric center on C/D rings.
The fascination for mastering the steroid skeleton still continues unabated.


