6.5: Interpreting C-13 NMR Spectra
- Page ID
- 432206
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- understand how chemical shift and number of peaks come together to determine functional groups.
- solve unknown 13C NMR problems given the molecular formula.
This section will help identify how to interpret information from 13C NMR spectra.
Starting with the 13C NMR spectrum for ethanol, C2H6O.

Remember that each peak identifies a carbon atom in a different environment within the molecule. In this case there are two peaks because there are two different environments for the carbons. The carbon in the CH3 group is attached to 3 hydrogens and a carbon. The carbon in the CH2 group is attached to 2 hydrogens, a carbon and an oxygen. So which peak is which?
You might remember from Section 6.2 that the external magnetic field experienced by the carbon nuclei is affected by the electronegativity of the atoms attached to them. The effect of this is that the chemical shift of the carbon increases if you attach a more electronegative atom like oxygen to it. That means that the peak at about 60 (the larger chemical shift) is due to the CH2 group because it has a more electronegative atom attached.
In principle, you should be able to work out the fact that the carbon attached to the oxygen will have the larger chemical shift. In practice, you always work from tables of chemical shift values for different groups (see below).
What if you needed to work it out? The electronegative oxygen pulls electrons away from the carbon nucleus leaving it more exposed to any external magnetic field. That means that you will need a smaller external magnetic field to bring the nucleus into the resonance condition than if it was attached to less electronegative things. The smaller the magnetic field needed, the higher the chemical shift.
A table of typical chemical shifts in 13C NMR spectra
| carbon environment | chemical shift (ppm) |
|---|---|
| C=O (in ketones) | 205 - 220 |
| C=O (in aldehydes) | 190 - 200 |
| C=O (in acids and esters) | 170 - 185 |
| C in aromatic rings | 125 - 150 |
| C=C (in alkenes) | 115 - 140 |
| RCH2OH | 50 - 65 |
| RCH2Cl | 40 - 45 |
| RCH2NH2 | 37 - 45 |
| R3CH | 25 - 35 |
| CH3CO- | 20 - 30 |
| R2CH2 | 16 - 25 |
| RCH3 | 10 - 15 |
In the table, the "R" groups will not necessarily be simple alkyl groups. If a substituent is very close to the carbon in question, and very electronegative, that might affect the values given in the table slightly. For example, ethanol has a peak at about 60 because of the -CH2OH group. No problem! It also has a peak due to the RCH3 group. The "R" group this time is -CH2OH. The electron pulling effect of the oxygen atom increases the chemical shift slightly from the one shown in the table to a value of about 18 ppm.
A simplification of the table:
| carbon environment | chemical shift (ppm) |
|---|---|
| C-C | 0 - 50 |
| C-O | 50 - 100 |
| C=C | 100 - 150 |
| C=O | 150 - 200 |
Now, we will look at 3-buten-2-one:
The structure for the compound is: . This molecule has carbons and all four are in different chemical environments. Therefore, in the 13C NMR spectrum there should be four signals.
The 13C NMR spectrum for 3-buten-2-one is:

Using the table above, you can assign each peak to each carbon.
- The peak at just under 200 ppm is due to a carbon-oxygen double bond. The two peaks at 137 ppm and 129 ppm are due to the carbons at either end of the carbon-carbon double bond. And the peak at 26 is the methyl group which, of course, is joined to the rest of the molecule by a carbon-carbon single bond. If you want to use the more accurate table, you have to put a bit more thought into it - and, in particular, worry about the values which do not always exactly match those in the table!
- The carbon-oxygen double bond in the peak for the ketone group has a slightly lower value than the table suggests for a ketone. There is an interaction (resonance) between the carbon-oxygen and carbon-carbon double bonds in the molecule which affects the value slightly. This will be observed in many conjugated systems. Discrepencies can also happen in more complicated systems.
- The two peaks for the carbons in the carbon-carbon double bond are exactly where they would be expected to be. Notice that they aren't in exactly the same environment, and so do not have the same shift values. The one closer to the carbon-oxygen double bond has the larger value.
- And the methyl group on the end has exactly the sort of value you would expect for one attached to C=O. The table gives a range of 20 - 30, and that's where it is.
One final important thing to notice. There are four carbons in the molecule, but they aren't all the same height. In 13C NMR, you cannot draw any simple conclusions from the heights of the various peaks.
Working out Structures from 13C NMR Spectra
So far, the structures of them molecules have been known and we have just been trying to see the relationship between carbons in particular environments in a molecule and the spectrum produced. Now let's make it a little more difficult - by looking at isomers! How could you tell from just a quick look at a 13C NMR spectrum whether you had propanone or propanal (assuming those were the only options)?
Because these are isomers, each has the same number of carbon atoms, but there is a difference between the environments of the carbons which will make a big impact on the spectra. In propanone, the two carbons in the methyl groups are in exactly the same environment, and so will produce only a single peak. That means that the propanone spectrum will have only 2 peaks - one for the methyl groups and one for the carbon in the C=O group. However, in propanal, all the carbons are in completely different environments, and the spectrum will have three peaks.
There are four alcohols with the molecular formula C4H10O.
A.
B.
C.
D.
Which one produced the 13C NMR spectrum below?

Solution
You can do this perfectly well without referring to chemical shift tables at all.
In the spectrum there are a total of three peaks - that means that there are only three different environments for the carbons, despite there being four carbon atoms.
In A and B, there are four totally different environments. Both of these would produce four peaks.
In D, there are only two different environments - all the methyl groups are exactly equivalent. D would only produce two peaks.
That leaves C. Two of the methyl groups are in exactly the same environment - attached to the rest of the molecule in exactly the same way. They would only produce one peak. With the other two carbon atoms, that would make a total of three. The alcohol is C.
This follows on from previous example, and also involves an isomer of C4H10O but which isn't an alcohol. Its 13C NMR spectrum is below. Work out what its structure is.

Solution
Because we do not know what sort of structure we are looking at, this time it would be a good idea to look at the shift values. The approximations are perfectly good, and we will work from this table:
| carbon environment | chemical shift (ppm) |
|---|---|
| C-C | 0 - 50 |
| C-O | 50 - 100 |
| C=C | 100 - 150 |
| C=O | 150 - 200 |
The peak at 66.75 ppm indicates there is a peak for carbon(s) in a carbon-oxygen single bond. The peak at 15.55 ppm indicates that there is a peak for carbon(s) in a carbon-carbon single bond. That would be consistent with C-C-O in the structure.
It is not an alcohol (you are told that in the question), and the molecular formula is C4H10O. With only two peaks, but four total carbons there must be symmetry in the carbons within the molecule. The only solution to that is to have two identical ethyl groups either side of the oxygen. The compound is ethoxyethane (diethyl ether), CH3CH2OCH2CH3.
Using the simplified table of chemical shifts above, work out the structure of the compound with the following 13C NMR spectrum. Its molecular formula is C4H6O2.
13C NMR Spectrum:
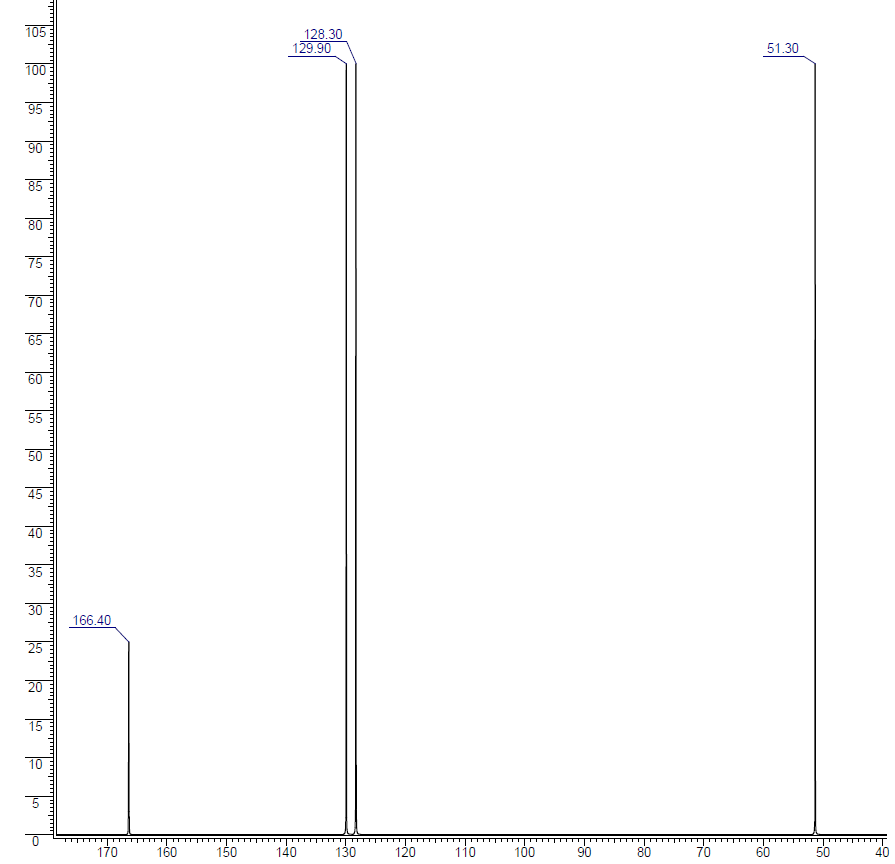
Solution
Let's sort out what we've got.
- There are four peaks and four carbons. No two carbons are in exactly the same environment, so all of our carbons are accounted for since there are only four in the molecular formula. This means no symmetry within the molecule.
- The peak at just over 50 must be a carbon attached to an oxygen by a single bond.
- The two peaks around 130 must be the two carbons at either end of a carbon-carbon double bond.
- The peak at just less than 170 is the carbon in a carbon-oxygen double bond.
Putting this together is a matter of playing around with the structures until you have come up with something reasonable. But you can't be sure that you have got the right structure using this simplified table. In this particular case, the spectrum was for the compound:
If you refer back to the more accurate table of chemical shifts towards the top of the page, you will get some better confirmation of this. The relatively low value of the carbon-oxygen double bond peak suggests an ester or acid rather than an aldehyde or ketone.
It can't be an acid because there has to be a carbon attached to an oxygen by a single bond somewhere - apart from the one in the -COOH group. We've already accounted for that carbon atom from the peak at about 170. If it was an acid, you would already have used up both oxygen atoms in the structure in the -COOH group. Without this information, though, you could probably come up with reasonable alternative structures. If you were working from the simplified table in an exam, your examiners would have to allow any valid alternatives.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)


