5.4: Types of Protons
- Page ID
- 432192
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to:
- identify those protons which are equivalent in a given chemical structure.
- use the 1H NMR spectrum of a simple organic compound to determine the number of equivalent sets of protons present.
Key Terms
Make certain that you can define, and use in context, the key terms below.
- diastereotopic
- enantiotopic
- homotopic
If all protons in all organic molecules had the same resonance frequency in an external magnetic field of a given strength, 1H NMR would not be terribly useful to organic chemists. Fortunately for organic chemists, resonance frequencies are not uniform for all protons in a molecule. In an external magnetic field of a given strength, protons in different locations in a molecule have different resonance frequencies, because they are in non-identical electronic environments. In methyl acetate, below for example, there would be two peaks in the 1H NMR spectrum, which means there are two types of protons. The three protons labeled Ha have a different - and easily distinguishable – resonance frequency than the three Hb protons, because the two sets of protons are in non-identical environments: they are, in other words, chemically nonequivalent.
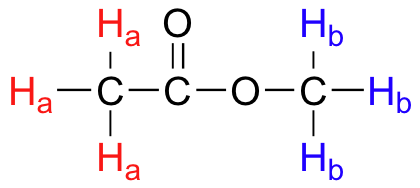
On the other hand, the three Ha protons are all in the same electronic environment, and are chemically equivalent to one another. They have identical resonance frequencies. The same can be said for the three Hb protons.
A good test to determine if hydrogens are chemically equivalent is by doing a thought exercise. In the thought exercise, you replace hydrogens by X to determine what the "thought molecules'" relationship would be to each other. If the protons in the two "thought molecules" are identical, then the protons are said to be homotopic. Homotopic protons are identical protons and will be chemically equivalent. This means that they will show up at the same location in the NMR spectrum.
Are the protons in methane, CH4, homotopic, enantiotopic, or diastereotopic?
Solution
Let's do the thought experiment. Two of the hydrogens in methane have been labeled a and b.
Now exhange replace each with X to form two separate "thought molecules". If we exchange Ha for X, then the molecule would be . Doing the same with Hb, the resulting molecule is
. The next step is to determine the relationship between these two molecules. Both "thought molecules" are identical, so the protons are homotopic.
You might expect that the equatorial and axial hydrogens in cyclohexane would be non-equivalent, and would have different resonance frequencies. In fact, an axial hydrogen is in a different electronic environment than an equatorial hydrogen. Remember, though, that the molecule rotates rapidly between its two chair conformations, meaning that any given hydrogen is rapidly moving back and forth between equatorial and axial positions. It turns out that, except at extremely low temperatures, this rotational motion occurs on a time scale that is much faster than the time scale of an NMR experiment.
In this sense, NMR is like a camera that takes photographs of a rapidly moving object with a slow shutter speed - the result is a blurred image. In NMR terms, this means that all 12 protons in cyclohexane are equivalent.
The next example we will consider is bromochloromethane. Are the protons of the CH2 chemically equivalent?
Are the protons in bromochloromethane, C2H4BrCl, homotopic, enantiotopic, or diastereotopic?
Solution
Start with the same thought experiment that we did with methane and exchange one of the hydrogens with X to make one "thought molecule" and then repeat with the other hydrogen. The two molecules you get are and
. The relationship between the two molecules is that they are enantiomers. These protons are considered enantiotopic.
In Example 5.4.2, the "thought" molecules were enantiomers of each other. The hydrogens are termed enantiotopic and like enantiomers the protons are only different in the presence of something that is chiral. The solvents typically used for NMR spectroscopy are achiral. Therefore, the two methylene protons are equivalent protons and will have the same chemical shift. For bromochloromethane, one would expect there to be one NMR absorption for the CH2 group. In summary, enantiotopic protons will be chemically equivalent. This means that they will show up at the same location in the NMR spectrum.
The final type of protons to discuss is diastereotopic protons.
Are the methylene protons in 2-bromo-2-chlorobutane, C4H8BrCl, chemically equivalent?
Solution
Start with the same thought experiment that we did with methane and exchange one of the hydrogens with X to make one "thought molecule" and then repeat with the other hydrogen. The two "thought molecules" are and
. The relationship between these two molecules is diastereomers. These protons are considered diastereotopic protons. Diastereomers have different chemical properties, which means these protons are not chemically equivalent.
Overall, 2-bromo-2-chlorobutane will have four different types of hydrogens: the two methyl groups will be different giving two NMR absorptions and the CH2 will give two NMR absorptions. In general, diastereotopic protons occur when there is a chirality center already present in the molecule. In summary, diastereotopic protons will be chemically different. This means that they will show up at different locations in the NMR spectrum.
When stereochemistry is taken into account, the issue of equivalence versus nonequivalence in NMR starts to get a little more complicated. It should be fairly intuitive that hydrogens on different sides of asymmetric ring structures and double bonds are in different electronic environments, and thus are nonequivalent and have different resonance frequencies. In the alkene and cyclohexane structures below, for example, Ha is trans to the chlorine substituent, while Hb is cis to chlorine. Ha and Hb in both the alkene and cyclohexane structures would give different absorptions in the NMR spectrum.
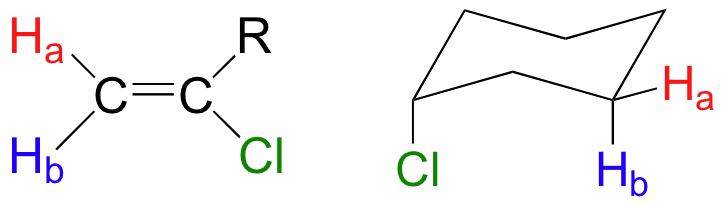
Most organic molecules have several sets of protons in different chemical environments, and each set, in theory, will have a different resonance frequency in 1H-NMR spectroscopy. The ability to recognize chemical equivalency and nonequivalency among atoms in a molecule will be central to understanding NMR.
Exercise
Are the labeled protons homotopic, enantiotopic, or diastereotopic?
a)
b)
- Answer
-
a) Diastereotopic
b) Enantiotopic
How many non-equivalent hydrogens are in the following molecules? How many different signals will you see in a 1H NMR spectrum?
a)
b)
c)
- Answer
-
a) 1-chloropropane has three non-equivalent hydrogens and would have 3 signals in an 1H NMR spectrum.
b) Diethylether has two non-equivalent hydrogens and would have 2 signals in an 1H NMR spectrum.
c) Ethylbenzene has five non-equivalent hydrogens and would have 5 signals in an 1H NMR spectrum.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
Contributors
- Derrick Kaseman (UC Davis), Sureyya OZCAN, Siyi Du

