9.2: Asymmetric Alkoxycarbonylation and Related Reactions
- Page ID
- 168823
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Reaction of Vinylarenes
From academic and industrial standpoint, the Pd-catalyzed asymmetric hydroxy- and alkoxycarbonylation reactions are attractive processes. However, they are less successful compared to the Rh-catalyzed hydroformylation reactions. This is because of the difficulty in getting simultaneously both high regio- and enantioselectivities.
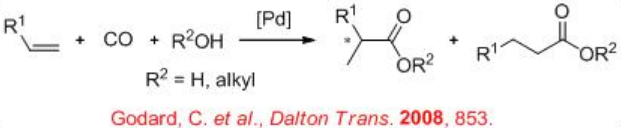
The alkoxycarbonylation of vinylarenes is an important process and the resulting products (2-arylpropanoic acids and derivatives) serve as substrate precursors for nonsteroidal anti-inflammatory drugs, particularly ibuprofen and naproxen (Scheme \(\PageIndex{1}\)).
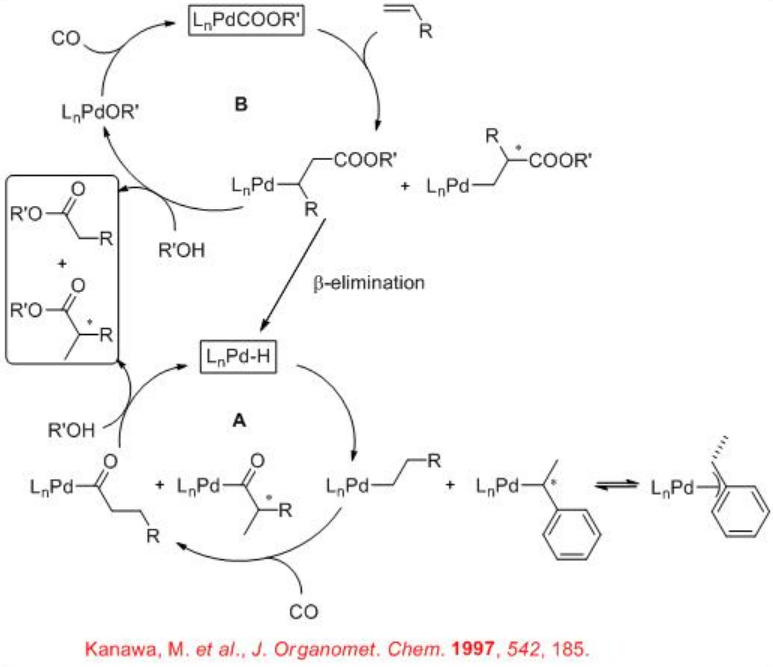
The coexistence of two catalytic cycles has been suggested for the alkoxycarbonylation reaction (Scheme \(\PageIndex{2}\)). In the hydrido-palladium complex cycle A, the insertion of the alkene to the Pd-H bond can give alkyl palladium complex that could react with CO via coordination and migratory insertion to yield Pd-acyl complex. Alcoholysis of the Pd-acyl complex can regenerate the Pd-H species and yield the ester. In the alkoxycarbonyl cycle B, the alkene inserts into the palladium-carbon bond of alkoxycarbonyl-palladium complex and the resulting product on alcoholysis gives an alkoxy-palladium complex and the ester. The alkoxy-palladium complex then reacts with CO via coordination and migratory insertion to regenerate the alkoxycarbonyl-palladium complex. The formation of the Pd-H species may also take place from the complexes formed in the catalytic cycle B via β -elimination of an unsaturated ester after the alkene insertion. In case of vinylarene, the branched alkyl intermediate could be stabilized through the formation of π -benzylic species.
In case of asymmetric synthesis, the regioselectivity of these reactions is of critical importance due to the branched products only contain the chiral center. Figure \(\PageIndex{1}\) summarizes the some of the diphosphine ligands used for the palladium catalyzed hydroxy- and alkoxycarbonylation of vinylarenes. Although the enantioselectivity is found to be moderate to good (up to 98%), in most of the methods, the regioselectivity is found to be low.
Bidentate pyridine-phosphine ligands have also been studied for palladium catalyzed asymmetric ethoxycarbonylation of styrene (Figure \(\PageIndex{2}\)). The regioselectivity of branched products is to be good but the enantioselectivity is found to be low (l/b = linear/branched).
Figure \(\PageIndex{3}\) summarizes the selective monodentate ligands studied for the palladium catalyzed asymmetric methoxycarbonylation of vinaylarenes. The ligand 18 is found to be effective for the hydroxycarbonylation of 2-vinyl-6-methoxynaphthalene under 1 atm of a mixture of CO and O2 in the presence of PdCl2 -CuCl2 at room temperature affording the target product with 91% ee and 100% regioselectivity.
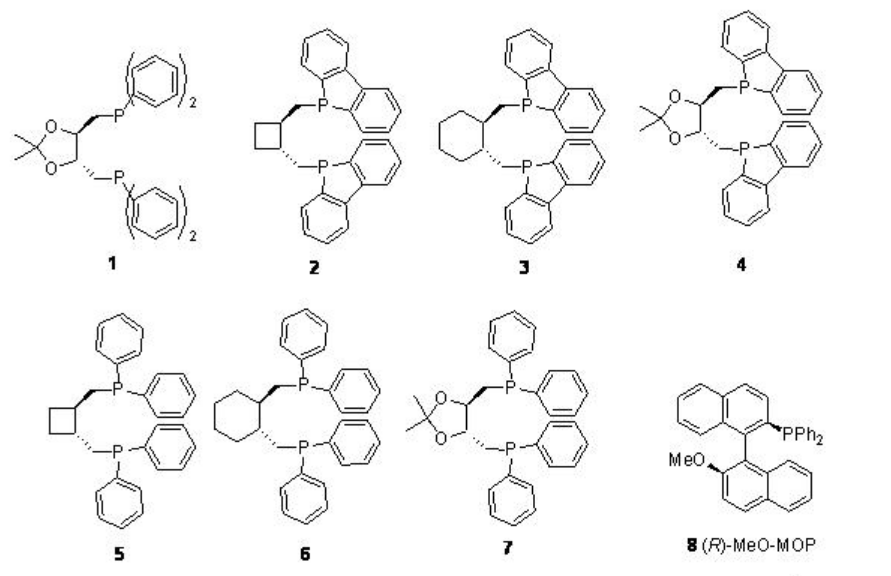
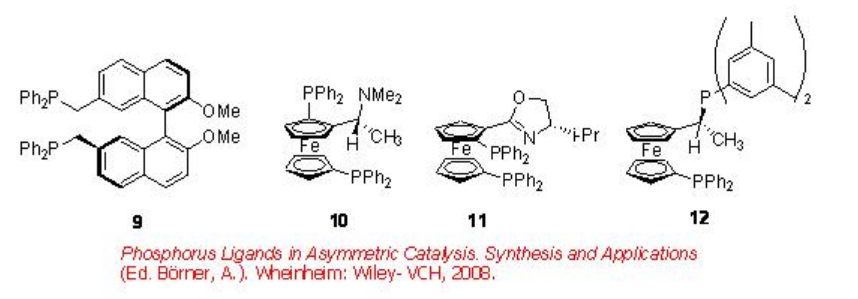
Figure \(\PageIndex{1}\): Diphosphine Ligands used in the Asymmetric Hydroxy- and Alkoxycarbonylation of Vinylarenes.
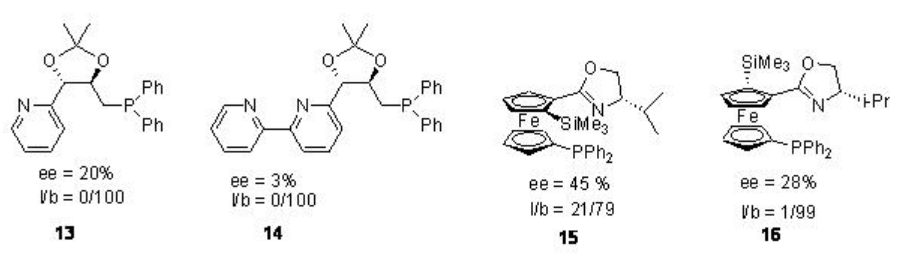
Figure \(\PageIndex{2}\): P-N Ligands used for Alkoxycarbonylation Reactions.
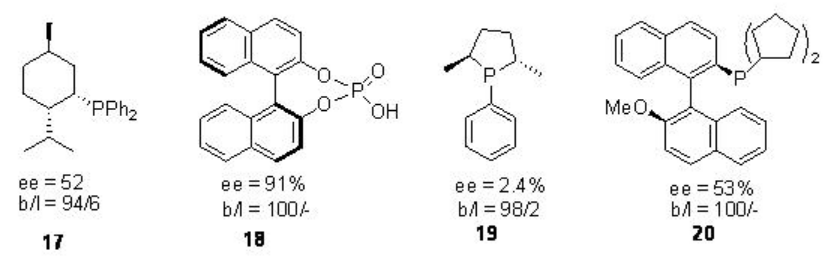
Figure \(\PageIndex{3}\): Monodentate Phosphine Ligands used for methoxcarbonylation of vinylarenes.
9.2.2 Reaction of Other Substrate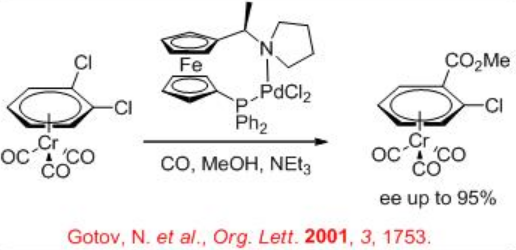
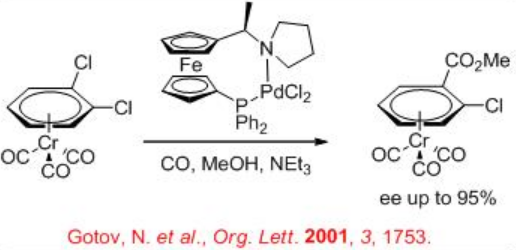
The methoxycarbonylation of 1,2-dichlorobenzene-Cr(CO)3 has been studied using palladium complex bearing chiral ferrocenyl ( R,S )-PPF-pyrrolidine system to introduce planar chirality in p -complexes (Scheme \(\PageIndex{3}\)). The reaction provides up to 95% ee in the presence 1 atm of CO at 60°C in the presence of triethylamine.
Bis-Alkoxycarbonylation of Vinylarenes
Optically active butanedioic acid derivatives are important class compounds that can be used as intermediates for the synthesis of pharmaceuticals and building blocks for the construction of inhibitors. Palladium-catalyzed bis-alkoxycarbonylation of alkenes provides effective methods for the construction of these compounds. In 1970, Heck reported the first example of the reaction and its asymmetric version appeared after nearly 20 years. Figure \(\PageIndex{4}\) summarizes some of the ligands employed for the bis-alkoxycarbonylation reactions. Chiral bidentate phosphine, P-N and S,N-ligands have been screened and high enantioselectivity (92%) is reported in the bis-methoxycarbonylation of styrene with moderate chemoselectivity (50%) employing 21 as the ligand. In case of propene, 60% ee is observed as the highest enantioselectivity with poor chemoselectivity and conversion (13% and 23%, respectively), while the reaction of 4-methyl-1-pentene afforded good chemoselectivity (79%) but with lower enantioselectivity (14% ee).
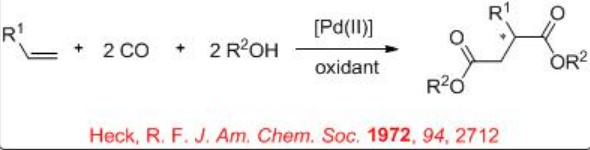
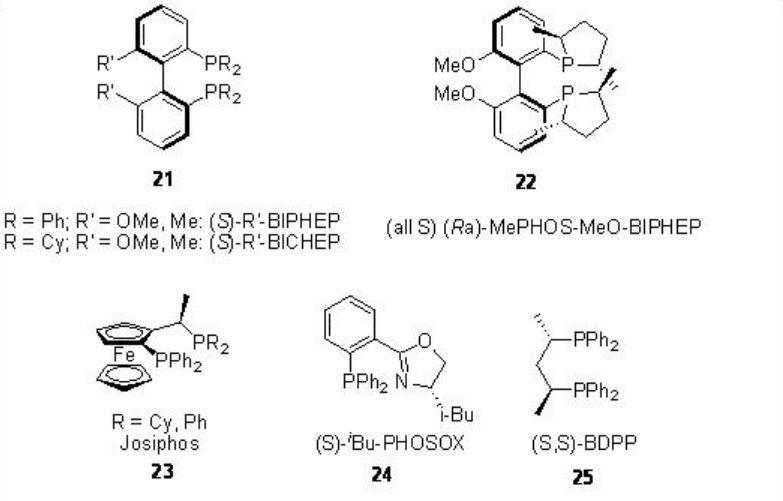
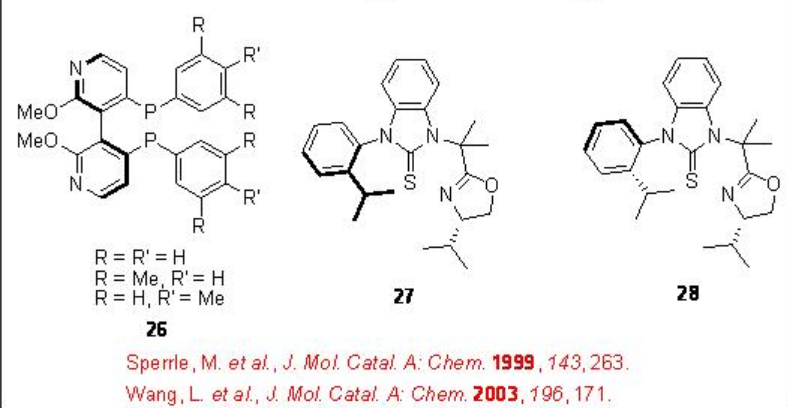
Figure \(\PageIndex{4}\): Ligands used in Asymmetric Bis-alkoxycarbonylation of Alkenes


