6.3: Reactions of Imines (C=N)
- Page ID
- 168803
An important field of investigation for new industrial catalysts is the development of improved catalysts for the reduction of imines to obtain the corresponding chiral amines. These chiral amines are used as key components in many active pharmaceutical intermediates.
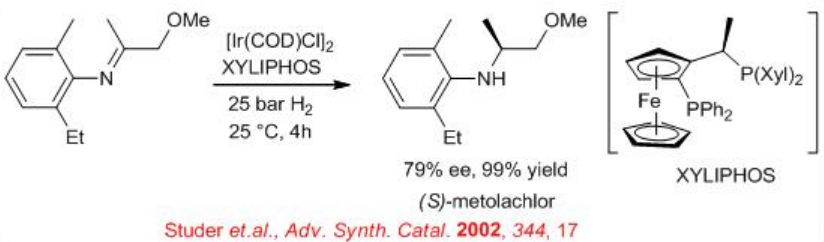
Synthesis of (S)-metolachlor (widely used as an herbicide) has been achieved by enantioselective hydrogenation of imine in presence of a catalyst generated in situ from [Ir(COD)Cl]2 and (R, S)-PPF–P(3,5-Xyl)2 (xyliphos) (Scheme \(\PageIndex{1}\)). This catalyst shows a high catalytic activity with TOF=396 h-1 and enantioselectivity of 79% ee.
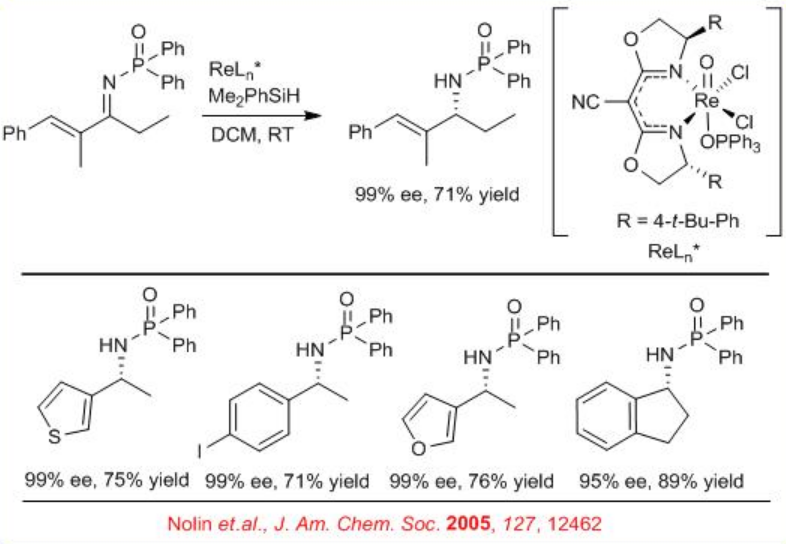
Subsequently, an air- and moisture-tolerant enantioselective reduction of N -phosphinyl imines has been performed with (CNbox)Re(O)Cl2 (OPPh3) (Scheme \(\PageIndex{2}\)). A wide range of aromatic imines, including cyclic, acyclic and heteroaromatic, α -iminoesters, and α,β -unsaturated imines undergo reaction with good to excellent enantioselectivity.
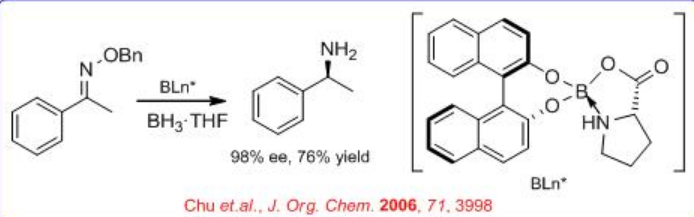
The use of modified CBS-type catalysts has been extended to the reduction of oximes into chiral amines (Scheme \(\PageIndex{3}\)). The BINOL-proline-borate complex reduces acetophenone oxime into chiral 1-phenylethylamine with 98% ee, but the ee drops when the borate complex is used catalytically.
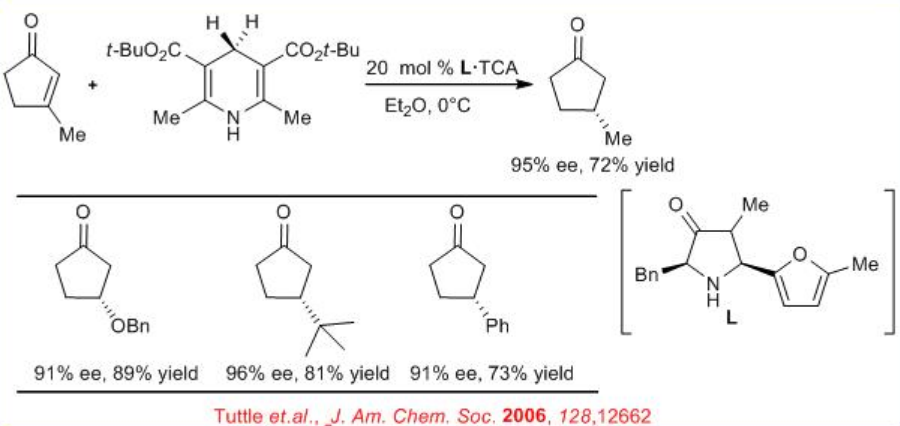
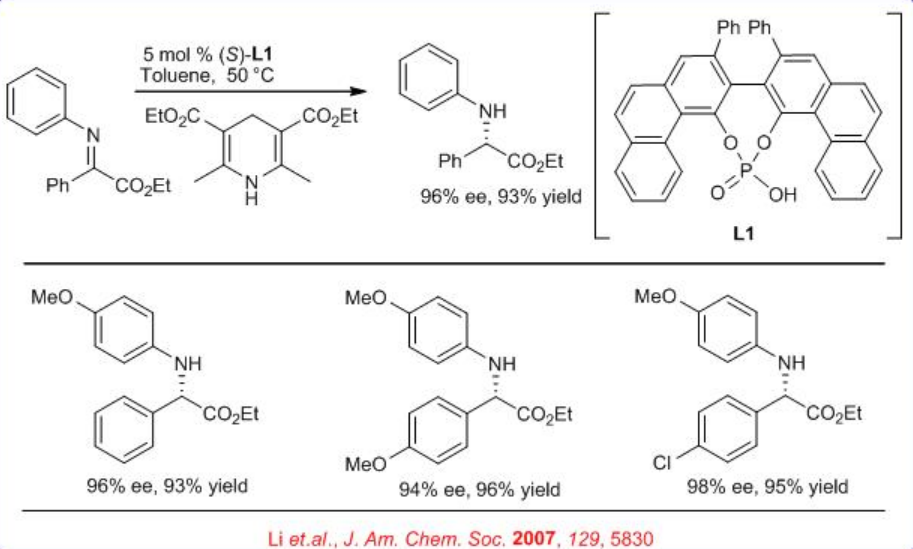
A new method for the reduction of α -imino esters using Hantzsch ester is reported with chiral phosphoric acid (Scheme 4). A series of α -imino esters could be reduced to the corresponding α-amino esters in excellent yield with up to 94% ee.
An efficient metal/brønsted acid relay catalysis has been shown for the highly enantioselective hydrogenation of quinoxalines through convergent disproportionation of dihydroquinoxalines with up to 94% (Scheme \(\PageIndex{5}\)).
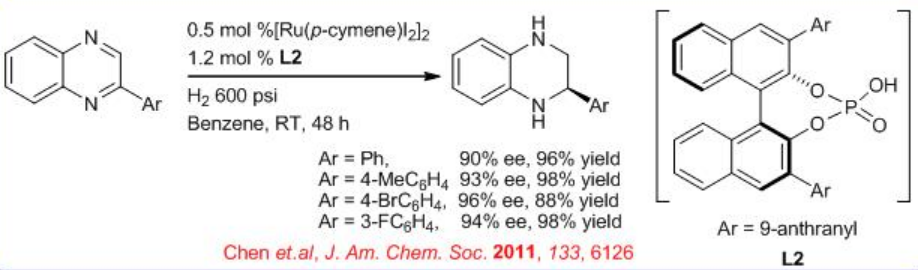
Employing hydrogen gas as the reductant makes this convergent disproportionation an ideal atom-economical process. A dramatic reversal of enantioselectivity is observed for the hydrogenation relative to the transfer hydrogenation of quinoxalines promoted by chiral phosphoric acids L2.
Asymmetric Transfer Hydrogenation Reactions (ATHRs)
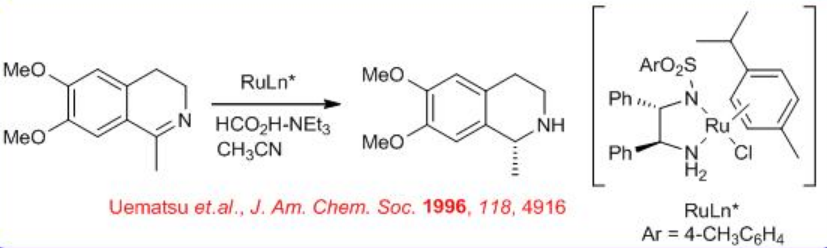
Another field where asymmetric transfer hydrogenation (ATH) catalysts have made an industrial impact is in the area of chiral amine synthesis by stereo controlled reduction of imines. The reduction of cyclic imines to yield chiral amines is proved to be a highly versatile and successful strategy for the synthesis of chiral tetrahydroisoquinolines and related compounds (Scheme \(\PageIndex{6}\)).
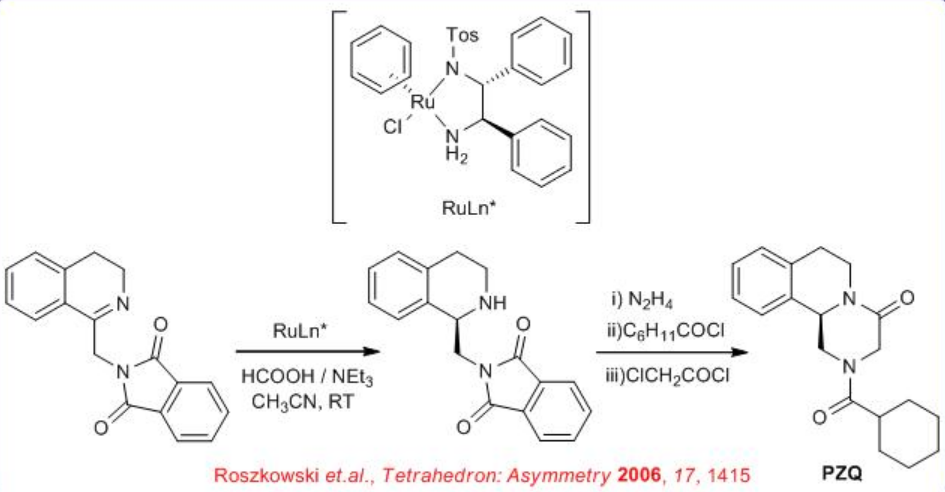
The enantioselective preparation of Praziquantel (PZQ) a pharmaceutical for the treatment of schistosomiasis and soil-transmitted helminthiasis has been accomplished. The synthesis is completed from staring chiral reduction of imine which could be synthesized from readily available phenyl ethyl amine, phthalic anhydride and glycine (Scheme \(\PageIndex{7}\)).
In parallel to metal catalysis, organo catalyst like chiral thiourea and chiral imidazoilidines have been used for the asymmetric hydrogen transfer (ATS) reaction in presence of Hantzsch ester. For example, enantioselective Hantzsch ester mediated conjugate transfer hydrogenation of α,β -disubstituted nitro-alkenes has been shown using chiral thiourea (Scheme \(\PageIndex{8}\)). A broad range of substrates including β,β -unsaturated aldehydes and ketones, ketimines and aldimines, α -keto esters, and now nitro alkenes are successfully employed for hydrogenation.
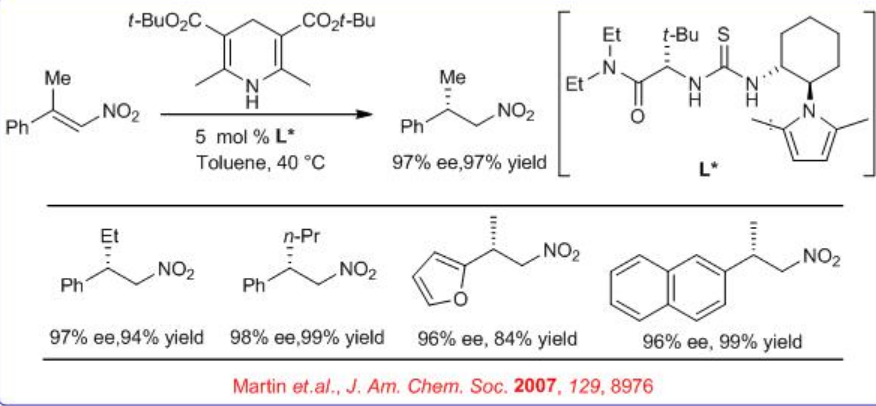
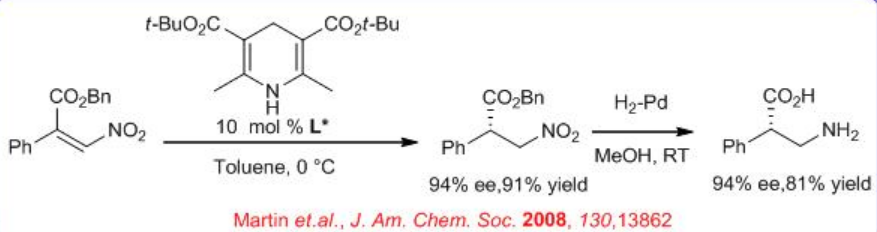
The above catalyst is also used for enantioselective Hantzsch ester mediated conjugate reduction of β -nitroacrylates (Scheme \(\PageIndex{9}\)). After subsequent reduction with Pd-H2-MeOH, chiral β -amino acids can be synthesized with high yield and ee. This provides a key step in a new route to optically active β2-amino acids.
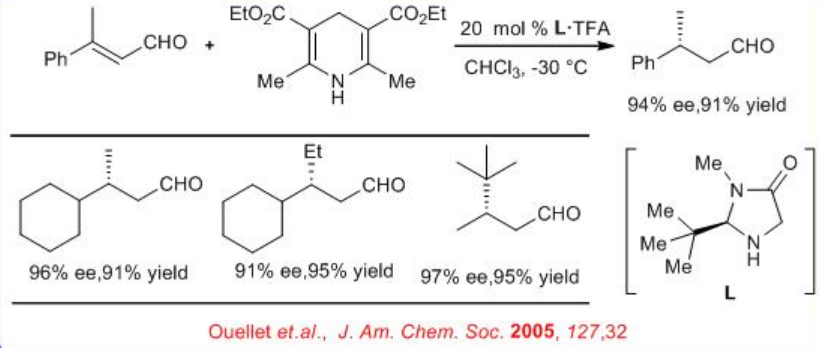
In parallel to the chiral thiourea catalyst, the use of iminium catalysis for the enantioselective reduction of β, β -substituted α, β -unsaturated aldehydes to generate β -stereogenic aldehydes has been shown (Scheme \(\PageIndex{10}\)). The capacity of the catalyst to accelerate ( E)-(Z) isomerization prior to selective ( E) -alkene reduction allows the implementation of geometrically impure enals in this operationally simple protocol.
The above catalytic system is used for transfer hydrogenation of cyclic enones (Scheme \(\PageIndex{11}\)). Cycloalkenones with 5-, 6-, and 7-membered ring systems undergo reaction with high stereoselectivity.