5.1: Oxidation of Alcohols
- Page ID
- 168794
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
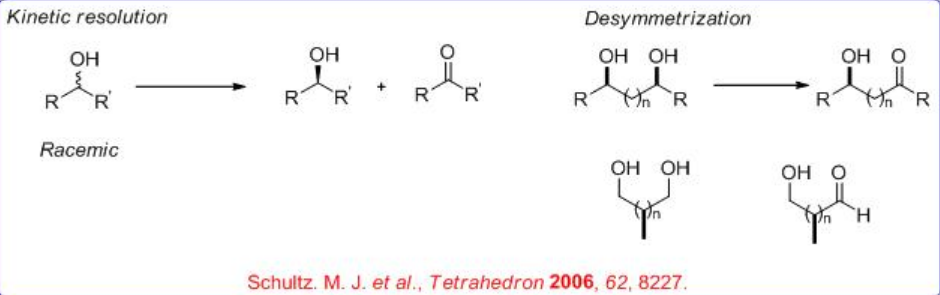
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Oxidation of alcohols to carbonyl compounds is a pivotal process in organic chemistry. In particular, the oxidations that use readily available molecular oxygen, especially ambient air, as the stoichiometric oxidant are the most preferable. During the recent years, asymmetric version of the process has been developed using molecular catalysis, which can be divided into kinetic resolution of secondary alcohols and desymmetrization of meso - or prochiral diols (Scheme \(\PageIndex{1}\)).
5.1.1 Palladium Catalyst
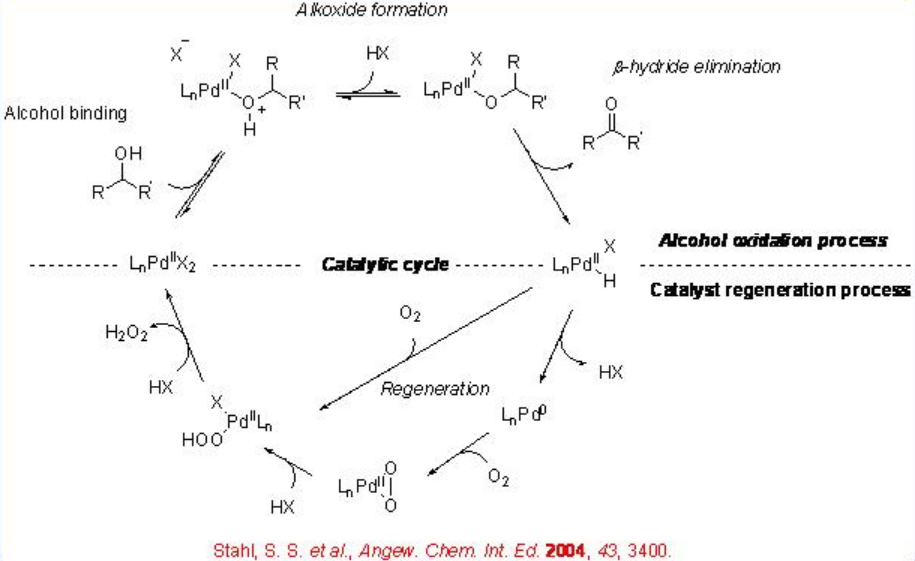
The palladium catalyzed aerobic oxidation of alcohols to carbonyl compounds has received much attention in recent years and the catalytic cycle for this process is presented in Scheme \(\PageIndex{2}\). The cycle consists two separate processes: the oxidation of alcohols and the regeneration of the catalyst. In the oxidation of alcohols palladium alkoxide is generated after the coordination of the alcohol, and then β -hydride elimination occurs to afford the carbonyl compounds. The resultant palladium hydride reacts with molecular oxygen to generate palladium hydroperoxo complex and the subsequent ligand exchange reproduce the catalyst.
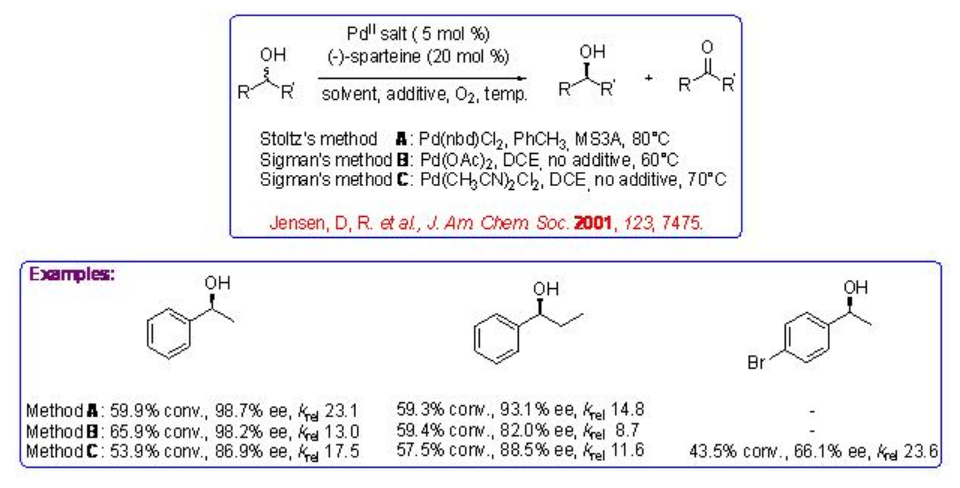
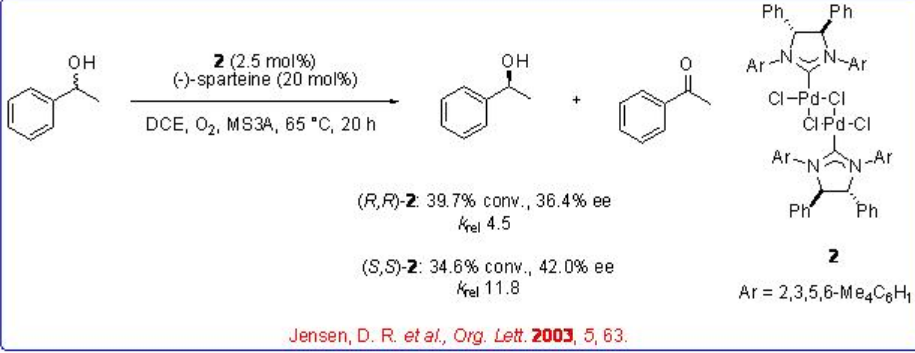
A series of experiments by three research groups have carried out using palladium complex bearing naturally occurring diamine, (-)-sparteine, to catalyzes the oxidation of aliphatic, benzylic and allylic alcohols with moderate to good krel values (Scheme \(\PageIndex{3}\)).
However, the isolated palladium-sparteine complex shows no catalytic activity and the reaction is effective employing additional (-)-sparteine (Scheme \(\PageIndex{4}\)). This result suggest that the additional (-)-sparteine serves as base to abstract a proton to a palladium bound alcohol in the alkoxide formation process.

Subsequently, the combination of palladium complexes bearing chiral and achiral N-heterocyclic carbene lignads with (-)-sparteine has been used for the kinetic resolution of secondary alcohols with high selectivity (Scheme \(\PageIndex{5}\)).
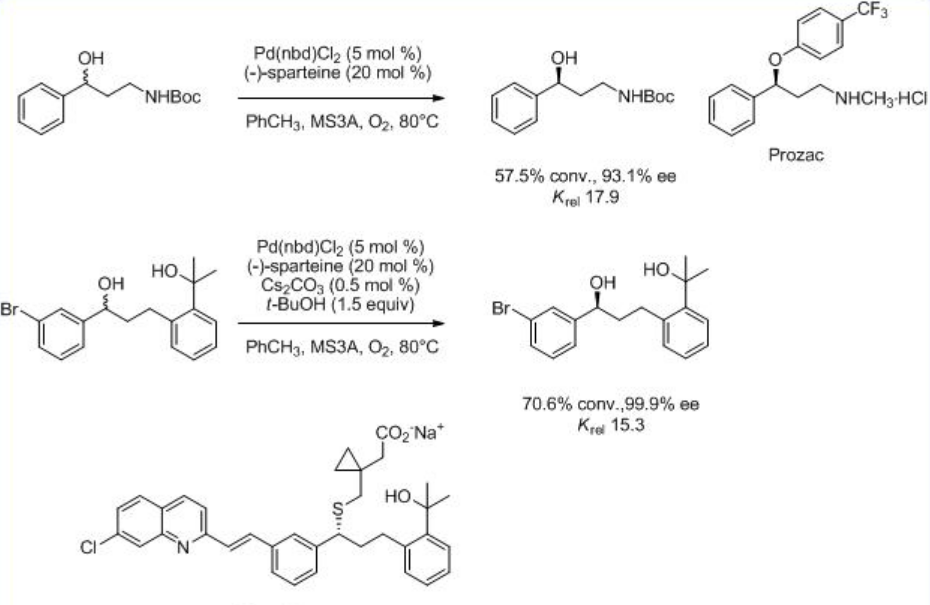
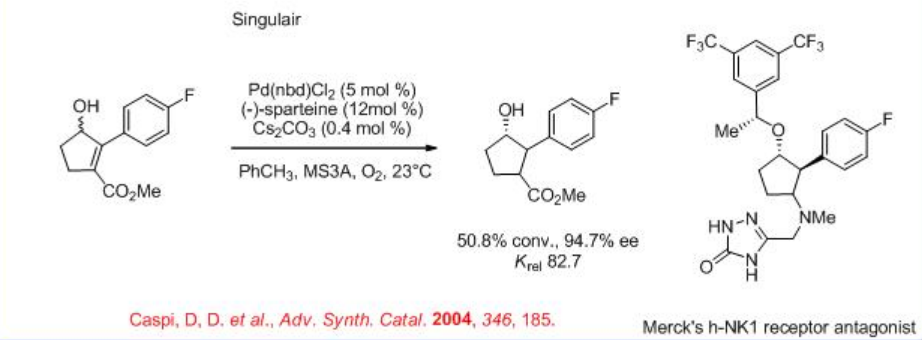
The reaction is found to be accelerated in the presence of Cs2CO3 under ambient air (Scheme \(\PageIndex{6}\)). The procedure is found to be useful for the synthesis of several pharmaceutically important substances including Prozac®, Singlair®, and Merck's h-NK1 receptor antagonist.
5.1.2 Ruthenium Catalyst
Chiral Ru-salen complex 3 having nitrosyl ligand has been found to be effective catalyst for the oxidative kinetic resolution of secondary alcohols under ambient air as oxidant under visible light (Scheme \(\PageIndex{7}\)). The irradiation of the visible light promotes dissociation of the nitrosyl ligand and generates a catalytically active ruthenium species. Kinetic resolution of aryl, alkynyl and alkyl alcohols has been observed with krel up to 30.
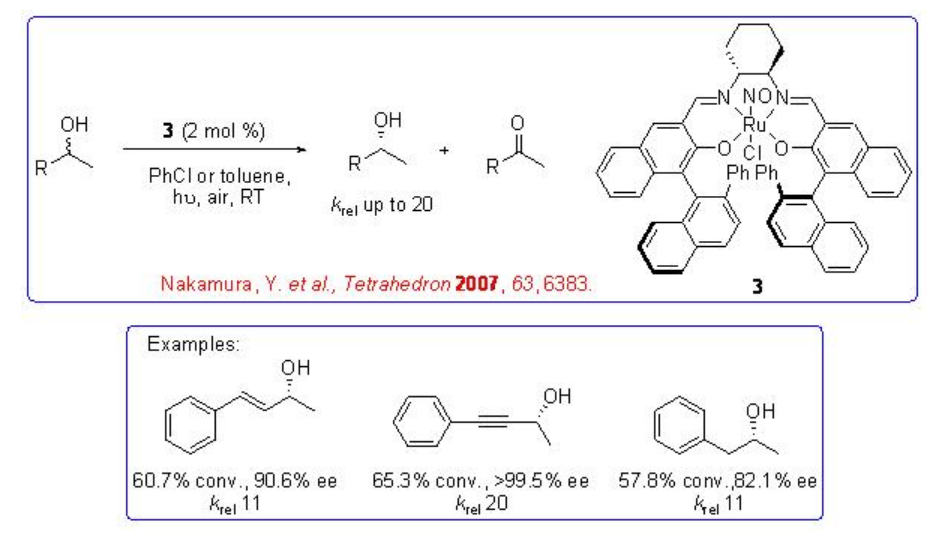
Scheme \(\PageIndex{7}\)
The chiral ruthenium based complex 4 is also effective for the oxidative desymmetrization of 1,4- meso -diols (Scheme \(\PageIndex{8}\)).
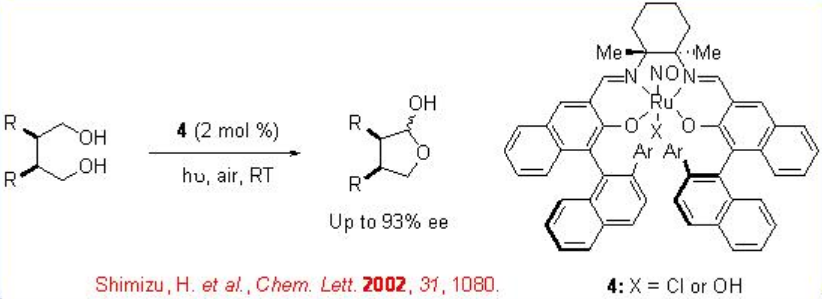
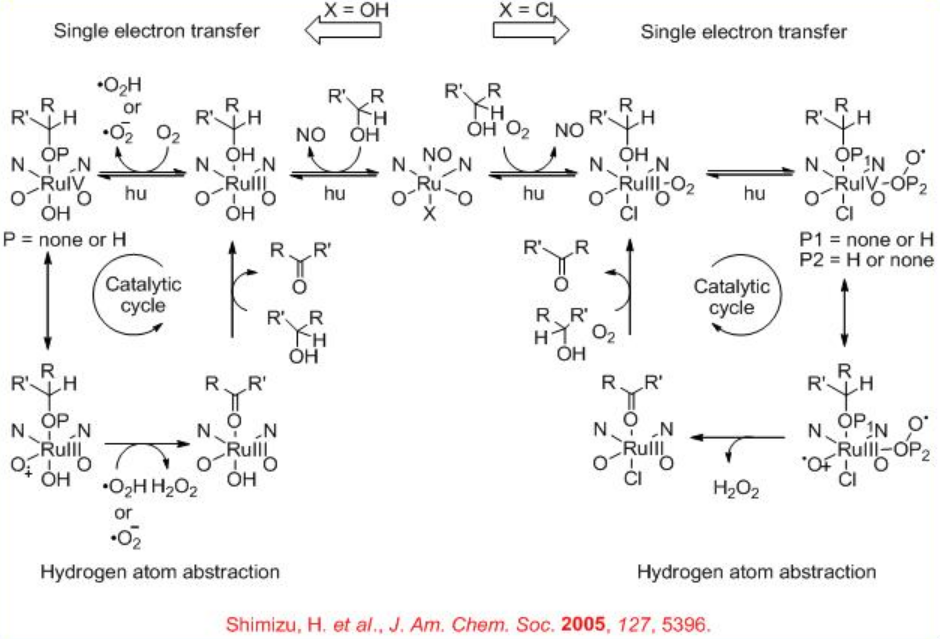
Scheme \(\PageIndex{9}\) shows the proposed mechanism for the Ru-catalyzed aerobic oxidation of alcohols which is similar to the galactose oxidase system.
5.1.3 Vanadium Catalyst
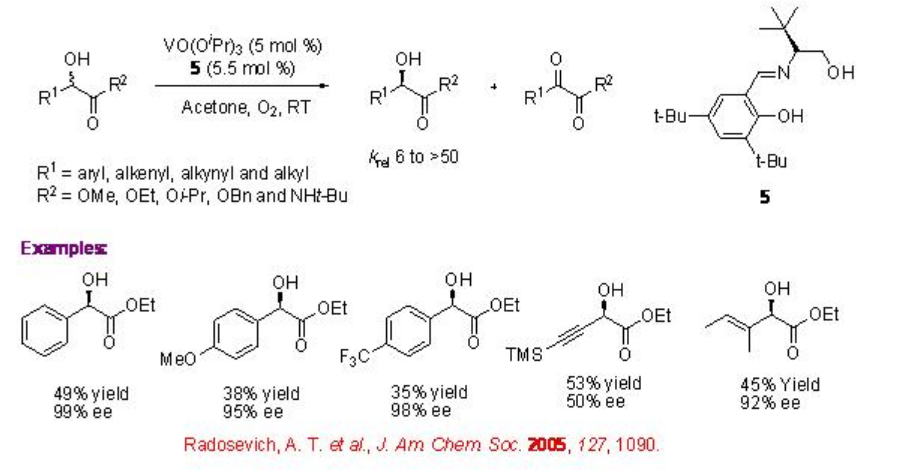
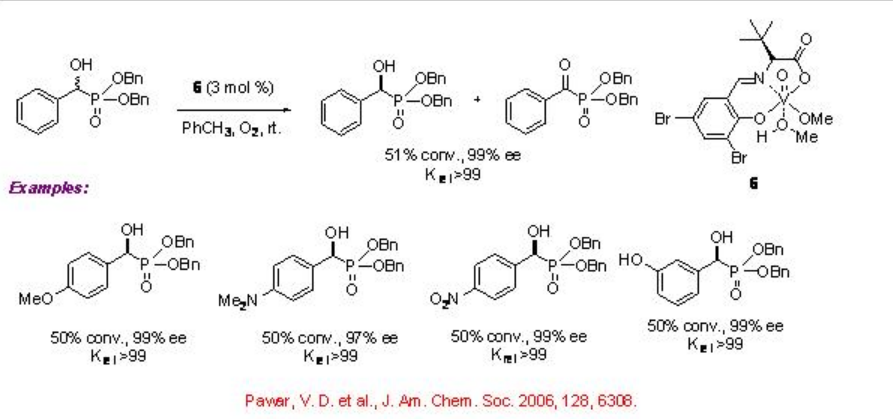
Vanadium complexes having chiral tridentate Schiff base ligand 5 derived from optically active amino alcohol and benzaldehyde derivative catalyze efficiently the kinetic resolution of α -hydroxy carbonyl compounds (Scheme \(\PageIndex{10}\)). The reactions of α -hydroxy esters can be accomplished with krelranging from 6 to 50. Subsequently, the chiral tridentate Schiff base ligand 6 derived from optically active α -amino acids and aldehydes have also been found to be effective for the vanadium catalyzed aerobic kinetic solution of hydroxy compounds (Scheme \(\PageIndex{11}\)). For example, the reaction of α -hydroxyphosphonic acids can be accomplished with excellent selectivity (krel 99). The observed experimental results suggest that these oxidation reactions don't involve radical process.
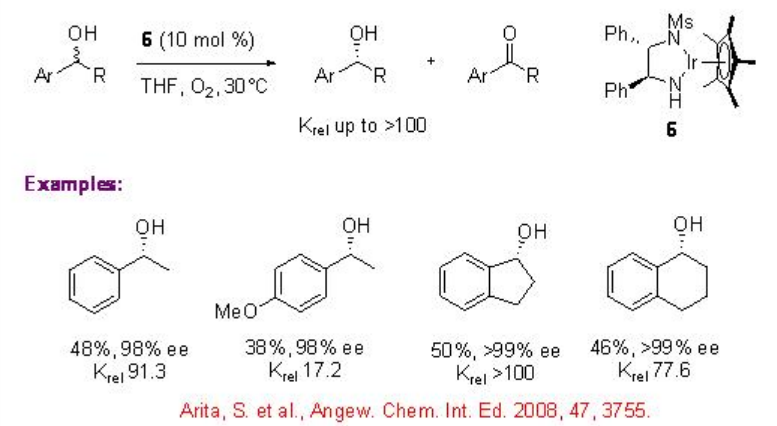
5.1.4 Iridium Catalyst
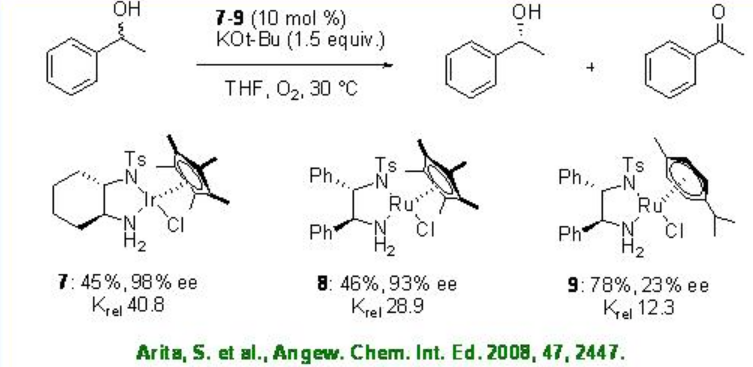
Few studies are focused on the use of chiral iridium complexes for the oxidative kinetic resolution of racemic secondary alcohols. Chiral iridium complex 6 has been shown to catalyze the oxidation benzylic alcohols with high krel under air. Using these reaction conditions, the oxidation of 1-indanol is reported with enantioselectivity of up to 99% and 50 yield.
Iridium chloride complex 7 has been used for the oxidation of racemic secondary alcohols with krelas high as 48.8 (Scheme \(\PageIndex{13}\)). The Rh analogue 8 exhibits high catalytic activity in the presence of base, while the related Ru complex 9 gives diminished result.