6.2: Reactions of Ketones
- Page ID
- 168802
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
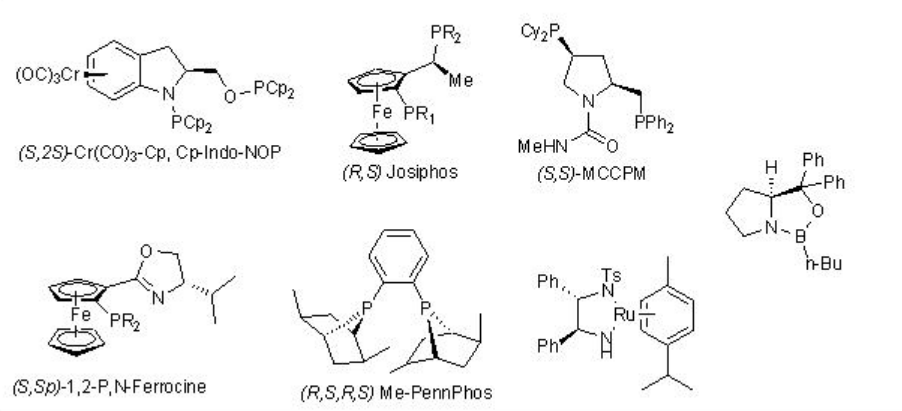
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Enantioselective reduction of C=O double bond in organic synthesis has important application in synthesis of many natural products as well as pharmaceutical products. The lecture covers the representative examples of metal catalyzed reactions. The reactions using CBS and enzymes are covered in the other modules of this course. The frequently used chiral ligands for the metal catalyzed enantioselective reduction reactions of ketones are listed in Scheme \(\PageIndex{1}\).
6.2.1 Reactions of α-Keto Amides
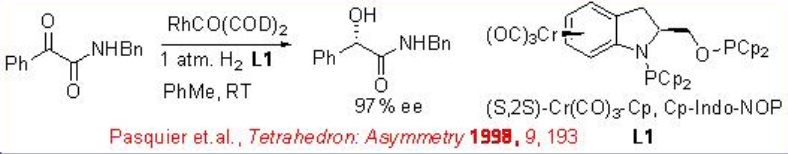
Asymmetric hydrogenation of α -keto esters has been studied with several rhodium catalysts. Neutral rhodium catalysts with chiral ligands such as Cr(CO)3-Cp,Cp-Indo-NOP demonstrate excellent enantioselectivity and reactivity in the hydrogenation of amides (Scheme \(\PageIndex{2}\)).
6.2.2 Reactions of β - Keto Esters
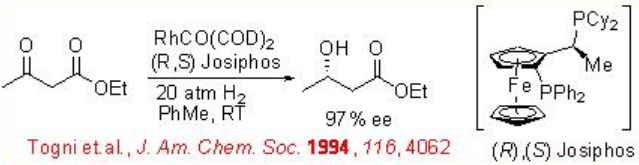
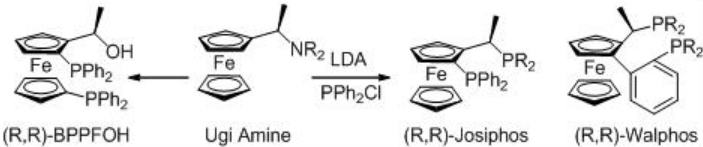
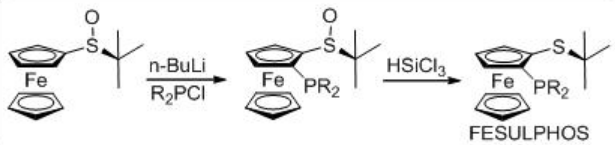
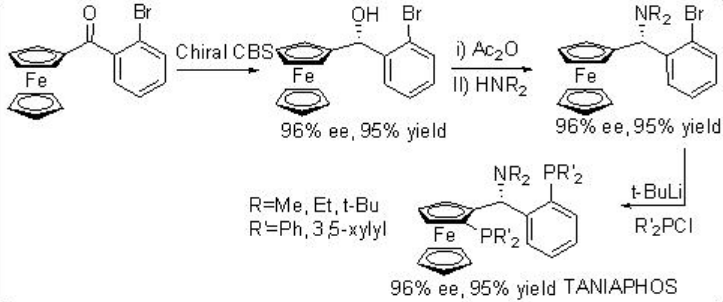
Asymmetric hydrogenation of β -keto esters has been extensively studied using chiral ruthenium catalysts. However, only handful of examples analogous to rhodium-catalyzed reaction are explored (Scheme 3). The Rh-( R,S )-Josiphos complex provides an effective catalyst for the asymmetric hydrogenation of ethyl 3-oxobutanoate affording the corresponding β -hydroxy ester in 97% ee. The above ligands Josiphos family such as chiral Walphos, Joshiphos, BPPFOH, TRAP and PIGIPHOS ligands could be easily synthesized from commercially available Ugi amine (Scheme \(\PageIndex{4}\)-\(\PageIndex{6}\)).
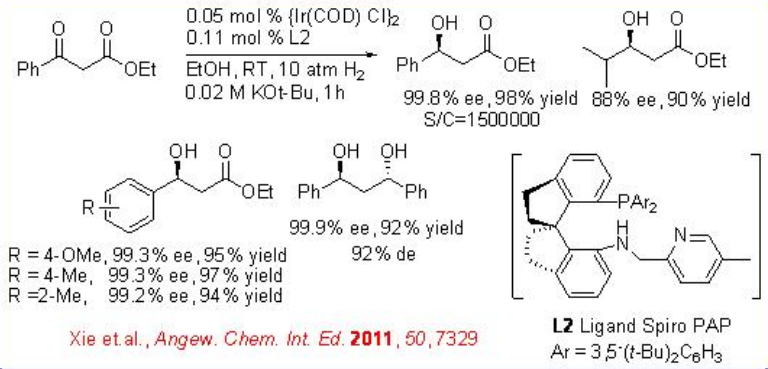
Iridium/spiro PAP has been used as effective catalyst for the asymmetric hydrogenation of β-aryl β-ketoesters (Scheme \(\PageIndex{7}\)). The reaction provides a readily accessible method for the synthesis of β-hydroxy esters in high enantioselectivity up to 99.8% ee and high TONs up to 1230000.
6.2.3 Reactions of Aromatic Ketones
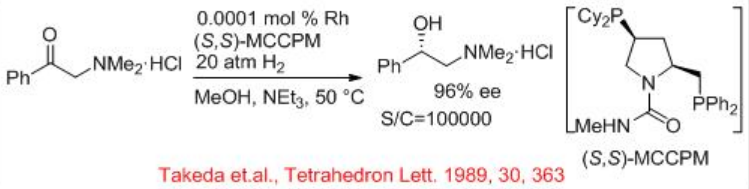
Amino ketones and their hydrochloride salts can be effectively hydrogenated with chiral rhodium catalysts (Scheme \(\PageIndex{8}\)). The rhodium precatalysts, combined with chiral phosphorous ligands (S,S)- MCCPM provide excellent enantioselectivity and reactivity for the asymmetric hydrogenation of α, β, and γ -alkyl amino ketone hydrochloride salts with S/C=100000.
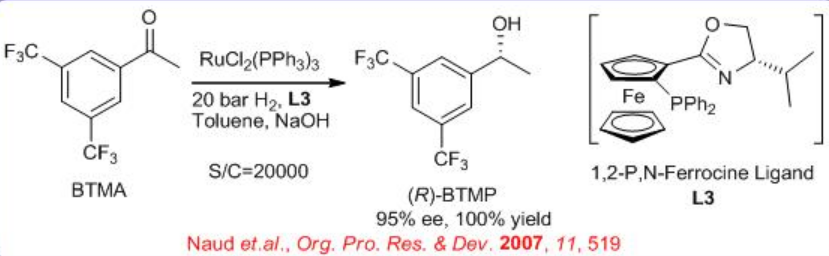
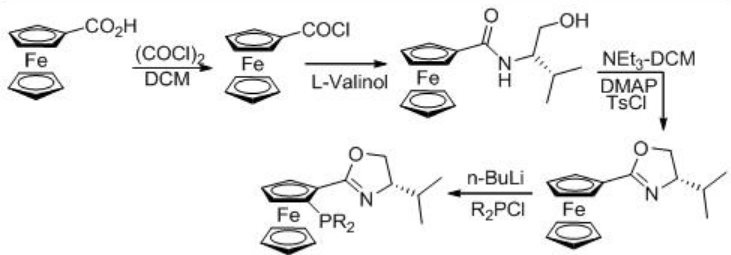
The enantioselective hydrogenation of 3,5-bistrifluoromethyl acetophenone (BTMA) can be carried out using a Ru/phosphine-oxazoline complex (Scheme \(\PageIndex{9}\)) . The reaction is compatible with 140-kg scale at 20 bar and 25°C with S/C ratios of 20,000. The synthesis of the ligand is shown in Scheme \(\PageIndex{10}\).
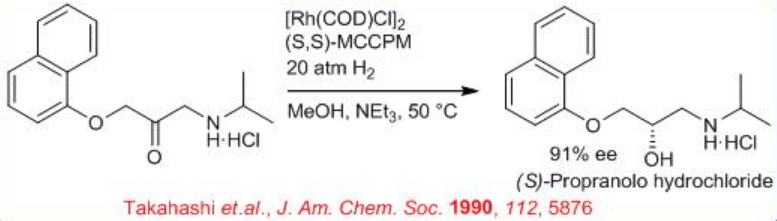
The enantioselective hydrogenation of amino ketones has been applied extensively to the synthesis of chiral drugs and pharmaceuticals (Scheme \(\PageIndex{11}\)). For example, direct enantioselective hydrogenation of 3-aryloxy-2-oxo-1-propylamine leads to 1-amino-3-aryloxy-2-propanol using 0.01 mol % of the neutral Rh-(S, S)-MCCPM complex. The chiral product 1-amino-3-aryloxy-2-propanol serves as β-adrenergic blocking agents. (S)-Propranolol is obtained in 90.8% ee from the corresponding α-amino ketone.
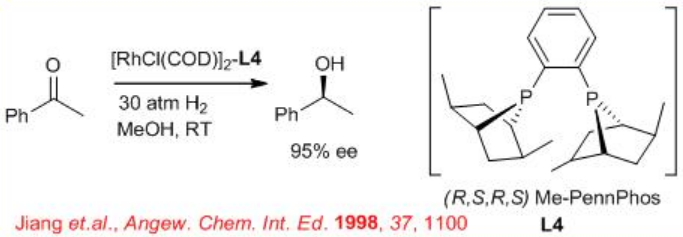
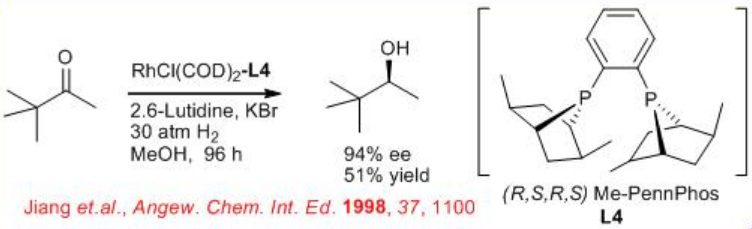
6.2.4 Reactions of Aliphatic Ketones
The asymmetric hydrogenation of simple aliphatic ketones remains still a challenging problem. This is due to the difficulty to design the appropriate chiral catalyst that will easily differentiate between the two-alkyl substituents of the ketone. Promising results have been obtained in asymmetric hydrogenation of aliphatic ketones using the ( R,S,R,S )-PennPhos- Rh complex in combination with 2,6-lutidine and KBr. For example, the reaction of tert -butyl methyl ketone takes place with 94% ee . Similarly, isopropyl-, n -butyl- and cyclohexyl methyl ketones can be reduced with 85% ee , 75% ee and 92% ee, respectively.
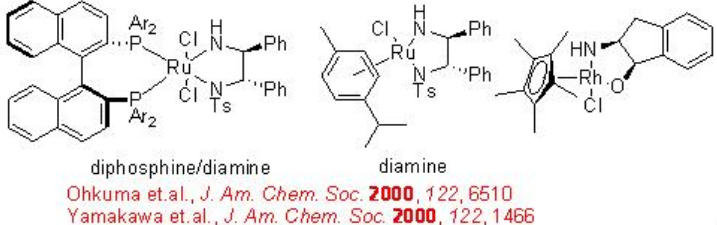
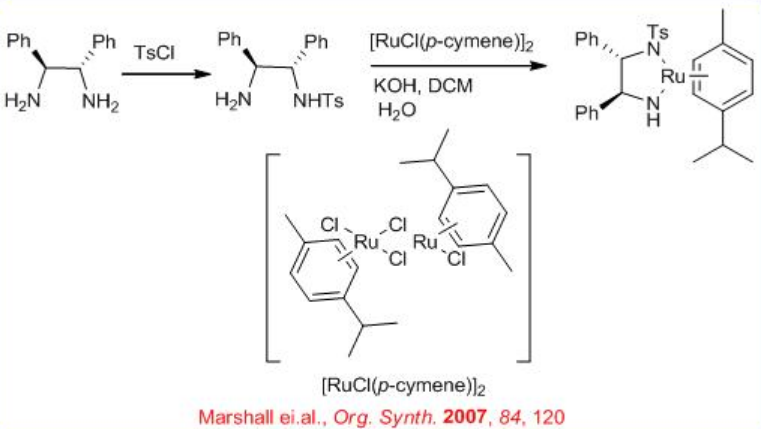
The chiral Ru-diphosphine/diamine derived from chiral BINAP, DPEN (diphenylethylene diamine) and indanol effect enantioselective hydrogenation of certain amino or amido ketones via a non-chelate mechanism without interaction between Ru and nitrogen or oxygen (Scheme \(\PageIndex{14}\)). The diamine catalyst can be synthesized from chiral 1,2- diphenylethylene diamine (Scheme \(\PageIndex{15}\)).
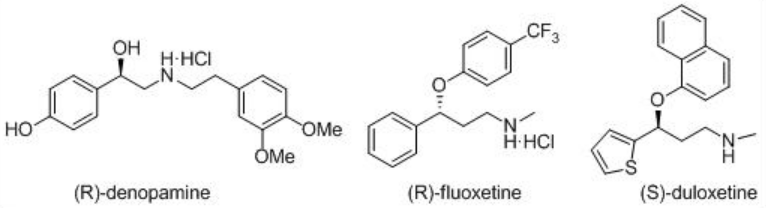
These catalysts have been employed for the asymmetric synthesis of various important pharmaceuticals, including (R)-denopamine, a β 1-receptor agonist, the anti -depressant (R)-fluoxetine, the anti -psychotic BMS 181100 and (S)-duloxetine (Scheme \(\PageIndex{16}\)).
Unsymmetric benzophenones could also be hydrogenated with high S/C ratio of up to 20000 without over-reduction (Scheme \(\PageIndex{17}\)). Enantioselective hydrogenation of certain ortho -substituted benzophenones leads to the unsymmetrically substituted benzhydrols, allowing convenient synthesis of the anti- cholinergic and anti -histaminic (S)-orphenadrine and antihistaminic (R)-neobenodine.
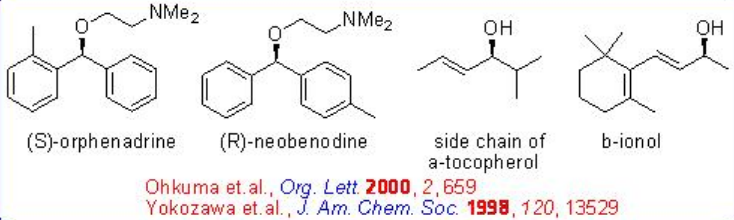
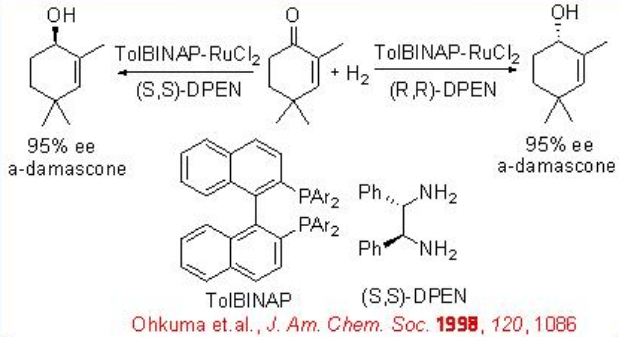
The asymmetric hydrogenation of simple ketone is generally achieved by the combined use of an (S)-BINAP and an (S)-1,2-diphenylethylenediamine. However, the reaction of 2,4,4-trimethyl-2-cyclohexenone can be effectively done with racemic RuCl2 [-tol-BINAP]- and chiral DPEN with up to >95% ee (Scheme \(\PageIndex{18}\)).