2.5: Allylic Substitution with Carbon Nucleophiles
- Page ID
- 168777
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The metal-catalyzed allylic substitution is one most of the important processes in organic synthesis. Scheme 1 represents the catalytic cycle of a transition metal based allylic substitution reaction. The reaction begins with the coordination of the low valent metal complex to the double bond of an allylic system. Subsequent oxidative addition by removal of the leaving group X gives a π -allyl complex as intermediate. The intermediate could be a neutral or cationic species, depending on the nature of the ligands and the counter ion X. The nucleophile typically adds to the terminal carbon with inversion of configuration rather than via the metal cation with retention (Scheme \(\PageIndex{1}\)).

Palladium-Catalyzed Reactions
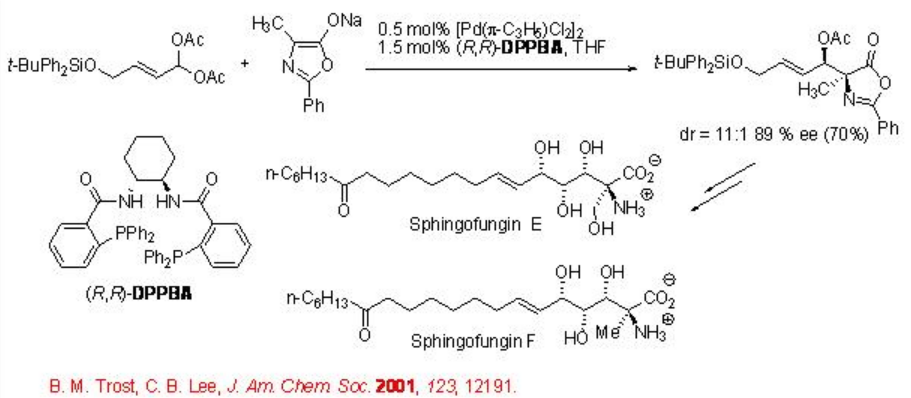
The palladium catalyzed allylic substitution reaction is a very powerful process. This section covers some recent examples on the palladium catalyzed enantioselective allylic substitution with carbon nucleophiles. The use of azlactones as a soft stabilized pronucleophile is particularly important because they give rise to amino acids as products. Scheme \(\PageIndex{2}\) presents Trost's synthesis of spingofungins via alkylation of a geminal diacetate with an azlactone. The product is formed with good diastereo- and enantioselectivity.
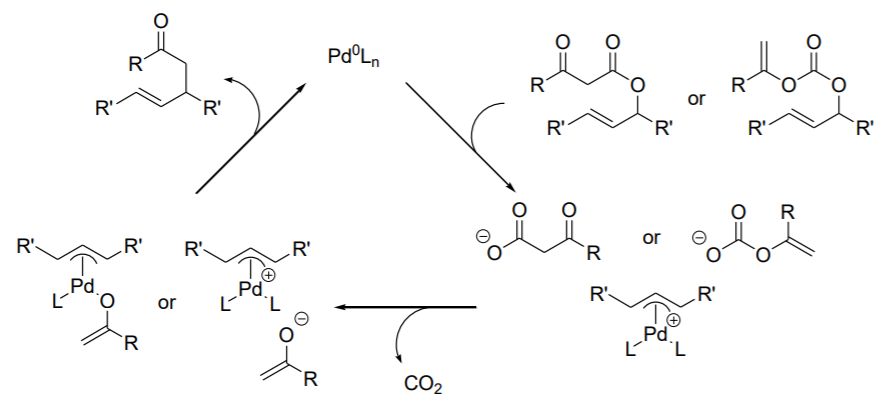
Atom economical method to obtain (π -allyl)Pd intermediates from allenes by addition of hydrido-Pd complexes has been demonstrated (Scheme \(\PageIndex{3}\)). This method affords the same products as that of the standard alkylation of allylic substrates. The pronucleophile are sufficiently acidic to produce HPdL2 species (Scheme \(\PageIndex{4}\)).


The palladium catalyzed reaction of vinyl epoxide with nucleophiles provides branched products (Scheme \(\PageIndex{5}\)). This is due to interaction of the nucleophile with an alkoxy or OH moiety produced by reaction with the Pd(0) species. For example, the reaction of isoprene monoepoxide with β-keto esters preferentially gives the branched alkylation products in the form of the hemiacetals (Scheme \(\PageIndex{6}\)). The nature of the β-ketoester and optimization of the reaction conditions are crucial for the success of this process.


Bimetallic system having Rh(acac)(CO)2, Pd(Cp)(π -C3H5) and the ligand Anis Trap has been used for the allylic alkylation with α -cyanopropionic acid derivative as pronucleophile (Scheme \(\PageIndex{7}\)). The control of the stereochemistry is believed to take place via the nucleophile with a chiral Rh complex coordinating to the cyano group.

Recently, allylic alkylation has been realized by enolate generated in situ by decarboxylation (Scheme \(\PageIndex{8}\)). Both allylic β -keto carboxylates and allyic enol carbonates undergo facile decarboxylation after oxidative addition of a Pd(0) species (Scheme \(\PageIndex{9}\)).

Nickel-Catalyzed Reactions
In comparison to the palladium catalyzed reactions, the nickel based chemistry is less explored. In addition, the nickel based chemistry less popular with the reactions of soft nucleophiles and few examples only so far investigated. For example, the reaction of allylic acetates has been studied with soft nucleophiles such as dimethyl malonate using a wide range of phosphine ligands (Scheme \(\PageIndex{10}\)). Linear allylic substrates give a mixture of regioisomers, whereas in cyclohexenyl acetate, the regioselectivity does not play any role affording the alkylated product with moderate enantioselectivity in the presence of chiral phosphine L1.

However, the nickel based systems are very popular with the reactions of hard nucleophiles such as boronic acids, borates and Grignard reagents. For example, the reaction of 1,3-disubstituted allyl ethers with Grignard reagents can be accomplished using nickel phosphine complex with good enantioselectivity (Scheme \(\PageIndex{11}\)). The reaction of methyl ether gave better results compared to phenyl ethers. In this reaction, if the reaction is quenched before complete consumption of the staring material, a significant kinetic resolution is observed.

Molybdenum-Catalyzed Reactions
Although the palladium catalyzed systems dominate in π-allyl chemistry, analogues Mo-catalyzed reactions have also emerged as powerful reactions in organic synthesis. The Mo-based reactions are the one first showed different regioselectivity compared to the palladium catalyzed systems. Scheme \(\PageIndex{12}\) illustrates the mechanism for the asymmetric Mo-catalyzed allylic alkylation.

Copper-Catalyzed Reactions
In case of the nonsymmetrical allylic substrates, the palladium catalyzed allylic alkylation reactions show poor regioselectivity. In this context, the copper based chemistry is an interesting alternative and lots of efforts have been made on this topic during last years. The copper based systems tolerate a wide range of hard and nonstabilized nucleophiles. Scheme \(\PageIndex{13}\) presents the regioselectivity in copper-catalyzed allylation reactions. In unsymmetrical substrates, nucleophile may attack directly at the leaving group (SN2) or at the allylic position (SN2’) under migration of the double bond depending on the reaction parameters as well as the substrate and nucleophile.


The observed results suggest that the regioselectivity and stereoselectivity are established at different stages (Scheme \(\PageIndex{14}\)). For example, the reaction of chiral carbamates with achiral copper reagent gives SN2’ product with excellent enantioselectivity (Scheme \(\PageIndex{15}\)).



